Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells
- PMID: 19406748
- PMCID: PMC2719395
- DOI: 10.1074/jbc.M109.013987
Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells
Abstract
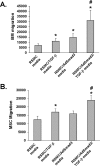
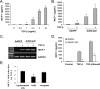
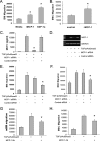
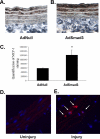
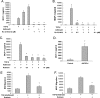
Bone marrow-derived progenitor cells have recently been shown to be involved in the development of intimal hyperplasia after vascular injury. Transforming growth factor-beta (TGF-beta) has profound stimulatory effects on intimal hyperplasia, but it is unknown whether these effects involve progenitor cell recruitment. In this study we found that although TGF-beta had no direct effect on progenitor cell recruitment, conditioned media derived from vascular smooth muscle cells (VSMC) stimulated with TGF-beta induced migration of both total bone marrow (BM) cells and BM-mesenchymal stem cells (MSC) and also induced MSC differentiation into smooth muscle like cells. Furthermore, overexpression of the signaling molecule Smad3 in VSMC via adenovirus-mediated gene transfer (AdSmad3) enhanced the TGF-beta's chemotactic effect. Microarray analysis of VSMC stimulated by TGF-beta/AdSmad3 revealed monocyte chemoattractant protein-1 (MCP-1) as a likely factor responsible for progenitor cell recruitment. We then demonstrated that TGF-beta through Smad3 phosphorylation induced a robust expression of MCP-1 in VSMC. Recombinant MCP-1 mimicked the stimulatory effect of conditioned media on BM and MSC migration. In the rat carotid injury model, Smad3 overexpression significantly increased MCP-1 expression after vascular injury, consistent with our in vitro results. Interestingly, TGF-beta/Smad3-induced MCP-1 was completely blocked by both Ro-32-0432 and rotterlin, suggesting protein kinase C-delta (PKCdelta) may play a role in TGF-beta/Smad3-induced MCP-1 expression. In summary, our data demonstrate that TGF-beta, through Smad3 and PKCdelta, stimulates VSMC production of MCP-1, which is a chemoattractant for bone marrow-derived cells, specifically MSC. Manipulation of this signaling system may provide a novel approach to inhibition of intimal hyperplasia.
Figures

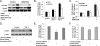

Similar articles
-
TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation.Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H540-9. doi: 10.1152/ajpheart.91478.2007. Epub 2009 Jun 12. Am J Physiol Heart Circ Physiol. 2009. PMID: 19525370 Free PMC article.
-
Transforming growth factor-β increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways.J Vasc Surg. 2012 Aug;56(2):446-54. doi: 10.1016/j.jvs.2011.12.038. Epub 2012 Apr 21. J Vasc Surg. 2012. PMID: 22521802 Free PMC article.
-
MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration.Blood. 2007 Feb 1;109(3):987-94. doi: 10.1182/blood-2006-07-036400. Epub 2006 Oct 10. Blood. 2007. PMID: 17032917
-
The role of progenitor cells in the development of intimal hyperplasia.J Vasc Surg. 2009 Feb;49(2):502-10. doi: 10.1016/j.jvs.2008.07.060. Epub 2008 Oct 22. J Vasc Surg. 2009. PMID: 18945574 Free PMC article. Review.
-
The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles.Stem Cell Rev. 2008 Sep;4(3):137-47. doi: 10.1007/s12015-008-9036-y. Epub 2008 Jul 30. Stem Cell Rev. 2008. PMID: 18665337 Free PMC article. Review.
Cited by
-
The Emerging Role of Mesenchymal Stem Cells in Vascular Calcification.Stem Cells Int. 2019 Apr 1;2019:2875189. doi: 10.1155/2019/2875189. eCollection 2019. Stem Cells Int. 2019. PMID: 31065272 Free PMC article. Review.
-
TGF-β mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells.Cancer Res. 2013 Apr 1;73(7):2333-44. doi: 10.1158/0008-5472.CAN-12-3086. Epub 2013 Jan 30. Cancer Res. 2013. PMID: 23365134 Free PMC article.
-
Tissue Engineering of Cartilage; Can Cannabinoids Help?Pharmaceuticals (Basel). 2010 Sep 6;3(9):2970-2985. doi: 10.3390/ph3092970. Pharmaceuticals (Basel). 2010. PMID: 27713386 Free PMC article. Review.
-
TGFβRI antagonist inhibits HIV-1 Nef-induced CC chemokine family ligand 2 (CCL2) in the brain and prevents spatial learning impairment.J Neuroinflammation. 2019 Dec 11;16(1):262. doi: 10.1186/s12974-019-1664-4. J Neuroinflammation. 2019. PMID: 31829243 Free PMC article.
-
TGF‑β/Smad signaling in chronic kidney disease: Exploring post‑translational regulatory perspectives (Review).Mol Med Rep. 2024 Aug;30(2):143. doi: 10.3892/mmr.2024.13267. Epub 2024 Jun 21. Mol Med Rep. 2024. PMID: 38904198 Free PMC article. Review.
References
-
- Newby A. C., Zaltsman A. B. ( 2000) J. Pathol. 190, 300– 309 - PubMed
-
- Ryer E. J., Hom R. P., Sakakibara K., Nakayama K. I., Nakayama K., Faries P. L., Liu B., Kent K. C. ( 2006) Arterioscler. Thromb. Vasc. Biol. 26, 780– 786 - PubMed
-
- Zernecke A., Schober A., Bot I., von Hundelshausen P., Liehn E. A., Möpps B., Mericskay M., Gierschik P., Biessen E. A., Weber C. ( 2005) Circ. Res. 96, 784– 791 - PubMed
-
- Massberg S., Konrad I., Schürzinger K., Lorenz M., Schneider S., Zohlnhoefer D., Hoppe K., Schiemann M., Kennerknecht E., Sauer S., Schulz C., Kerstan S., Rudelius M., Seidl S., Sorge F., Langer H., Peluso M., Goyal P., Vestweber D., Emambokus N. R., Busch D. H., Frampton J., Gawaz M. ( 2006) J. Exp. Med. 203, 1221– 1233 - PMC - PubMed
-
- Wang C. H., Anderson N., Li S. H., Szmitko P. E., Cherng W. J., Fedak P. W., Fazel S., Li R. K., Yau T. M., Weisel R. D., Stanford W. L., Verma S. ( 2006) Circ. Res. 99, 617– 625 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

