Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration
- PMID: 19403822
- PMCID: PMC6665849
- DOI: 10.1523/JNEUROSCI.0759-09.2009
Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration
Abstract
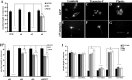
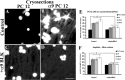
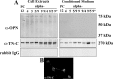
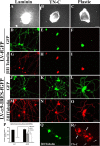
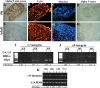
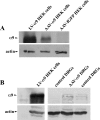
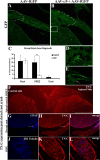
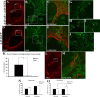
Damaged CNS axons are prevented from regenerating by an environment containing many inhibitory factors. They also lack an integrin that interacts with tenascin-C, the main extracellular matrix glycoprotein of the CNS, which is upregulated after injury. The alpha9beta1 integrin heterodimer is a receptor for the nonalternatively spliced region of tenascin-C, but the alpha9 subunit is absent in adult neurons. In this study, we show that PC12 cells and adult rat dorsal root ganglion (DRG) neurons do not extend neurites on tenascin-C. However, after forced expression of alpha9 integrin, extensive neurite outgrowth from PC12 cells and adult rat DRG neurons occurs. Moreover, both DRG neurons and PC12 cells secrete tenascin-C, enabling alpha9-transfected cells to grow axons on tissue culture plastic. Using adeno-associated viruses to express alpha9 integrin in vivo in DRGs, we examined axonal regeneration after cervical dorsal rhizotomy or dorsal column crush in the adult rat. After rhizotomy, significantly more dorsal root axons regrew into the dorsal root entry zone at 6 weeks after injury in alpha9 integrin-expressing animals than in green fluorescent protein (GFP) controls. Similarly, after a dorsal column crush injury, there was significantly more axonal growth into the lesion site compared with GFP controls at 6 weeks after injury. Behavioral analysis after spinal cord injury revealed that both experimental and control groups had an increased withdrawal latency in response to mechanical stimulation when compared with sham controls; however, in response to heat stimulation, normal withdrawal latencies returned after alpha9 integrin treatment but remained elevated in control groups.
Figures
Similar articles
-
Expression of an Activated Integrin Promotes Long-Distance Sensory Axon Regeneration in the Spinal Cord.J Neurosci. 2016 Jul 6;36(27):7283-97. doi: 10.1523/JNEUROSCI.0901-16.2016. J Neurosci. 2016. PMID: 27383601 Free PMC article.
-
Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent.eNeuro. 2016 Jul 27;3(4):ENEURO.0029-16.2016. doi: 10.1523/ENEURO.0029-16.2016. eCollection 2016 Jul-Aug. eNeuro. 2016. PMID: 27570822 Free PMC article.
-
Integrin-Driven Axon Regeneration in the Spinal Cord Activates a Distinctive CNS Regeneration Program.J Neurosci. 2023 Jun 28;43(26):4775-4794. doi: 10.1523/JNEUROSCI.2076-22.2023. Epub 2023 Jun 5. J Neurosci. 2023. PMID: 37277179 Free PMC article.
-
An integrin approach to axon regeneration.Eye (Lond). 2017 Feb;31(2):206-208. doi: 10.1038/eye.2016.293. Epub 2016 Dec 23. Eye (Lond). 2017. PMID: 28009347 Free PMC article. Review.
-
Netrin-1 signaling for sensory axons: Involvement in sensory axonal development and regeneration.Cell Adh Migr. 2009 Apr-Jun;3(2):171-3. doi: 10.4161/cam.3.2.7837. Epub 2009 Apr 14. Cell Adh Migr. 2009. PMID: 19262170 Free PMC article. Review.
Cited by
-
Role of immune responses for extracellular matrix remodeling in the ischemic brain.Ther Adv Neurol Disord. 2018 Dec 17;11:1756286418818092. doi: 10.1177/1756286418818092. eCollection 2018. Ther Adv Neurol Disord. 2018. PMID: 30619510 Free PMC article. Review.
-
Anti-integrin monoclonal antibodies.J Cell Sci. 2009 Nov 15;122(Pt 22):4009-11. doi: 10.1242/jcs.056770. J Cell Sci. 2009. PMID: 19910492 Free PMC article. Review. No abstract available.
-
Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC.Dev Neurobiol. 2010 Oct;70(12):826-41. doi: 10.1002/dneu.20820. Dev Neurobiol. 2010. PMID: 20629049 Free PMC article.
-
Co-targeting B-RAF and PTEN Enables Sensory Axons to Regenerate Across and Beyond the Spinal Cord Injury.Front Mol Neurosci. 2022 Apr 26;15:891463. doi: 10.3389/fnmol.2022.891463. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35557554 Free PMC article.
-
L-serine treatment may improve neurorestoration of rats after permanent focal cerebral ischemia potentially through improvement of neurorepair.PLoS One. 2014 Mar 26;9(3):e93405. doi: 10.1371/journal.pone.0093405. eCollection 2014. PLoS One. 2014. PMID: 24671106 Free PMC article.
References
-
- Bartsch U. The extracellular matrix molecule tenascin-C: expression in vivo and functional characterization in vitro. Prog Neurobiol. 1996;49:145–168. - PubMed
-
- Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
