A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2
- PMID: 19403670
- PMCID: PMC2704796
- DOI: 10.1128/JVI.00153-09
A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2
Abstract
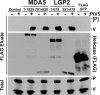
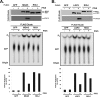
Diverse members of the Paramyxovirus family of negative-strand RNA viruses effectively suppress host innate immune responses through the actions of their V proteins. The V protein mediates interference with the interferon regulatory RNA helicase MDA5 to avoid cellular antiviral responses. Analysis of the interaction interface revealed the MDA5 helicase C domain as necessary and sufficient for association with V proteins from human parainfluenza virus type 2, parainfluenza virus type 5, measles virus, mumps virus, Hendra virus, and Nipah virus. The identified approximately 130-residue region is highly homologous between MDA5 and the related antiviral helicase LGP2, but not RIG-I. Results indicate that the paramyxovirus V proteins can also associate with LGP2. The V protein interaction was found to disrupt ATP hydrolysis mediated by both MDA5 and LGP2. These findings provide a potential mechanistic basis for V protein-mediated helicase interference and identify LGP2 as a second cellular RNA helicase targeted by paramyxovirus V proteins.
Figures

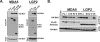
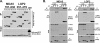

Similar articles
-
Paramyxovirus V protein interaction with the antiviral sensor LGP2 disrupts MDA5 signaling enhancement but is not relevant to LGP2-mediated RLR signaling inhibition.J Virol. 2014 Jul;88(14):8180-8. doi: 10.1128/JVI.00737-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829334 Free PMC article.
-
Amino acid requirements for MDA5 and LGP2 recognition by paramyxovirus V proteins: a single arginine distinguishes MDA5 from RIG-I.J Virol. 2013 Mar;87(5):2974-8. doi: 10.1128/JVI.02843-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269789 Free PMC article.
-
Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2.J Biol Chem. 2009 Apr 10;284(15):9700-12. doi: 10.1074/jbc.M807365200. Epub 2009 Feb 11. J Biol Chem. 2009. PMID: 19211564 Free PMC article.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
-
Retinoic acid-inducible gene-I-like receptors.J Interferon Cytokine Res. 2011 Jan;31(1):27-31. doi: 10.1089/jir.2010.0057. Epub 2010 Oct 15. J Interferon Cytokine Res. 2011. PMID: 20950133 Review.
Cited by
-
Insights into antiviral innate immunity revealed by studying hepatitis C virus.Cytokine. 2015 Aug;74(2):190-7. doi: 10.1016/j.cyto.2015.03.007. Epub 2015 Mar 25. Cytokine. 2015. PMID: 25819428 Free PMC article. Review.
-
MDA5 and LGP2: accomplices and antagonists of antiviral signal transduction.J Virol. 2014 Aug;88(15):8194-200. doi: 10.1128/JVI.00640-14. Epub 2014 May 21. J Virol. 2014. PMID: 24850739 Free PMC article. Review.
-
Paramyxovirus V protein interaction with the antiviral sensor LGP2 disrupts MDA5 signaling enhancement but is not relevant to LGP2-mediated RLR signaling inhibition.J Virol. 2014 Jul;88(14):8180-8. doi: 10.1128/JVI.00737-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829334 Free PMC article.
-
Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus.PLoS Pathog. 2018 Jun 29;14(6):e1007135. doi: 10.1371/journal.ppat.1007135. eCollection 2018 Jun. PLoS Pathog. 2018. PMID: 29958302 Free PMC article.
-
A Comparative Assessment of the Pathogenic Potential of Newly Discovered Henipaviruses.Pathogens. 2024 Jul 16;13(7):587. doi: 10.3390/pathogens13070587. Pathogens. 2024. PMID: 39057814 Free PMC article. Review.
References
-
- Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. - PubMed
-
- Cordin, O., J. Banroques, N. K. Tanner, and P. Linder. 2006. The DEAD-box protein family of RNA helicases. Gene 36717-37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

