ENaC at the cutting edge: regulation of epithelial sodium channels by proteases
- PMID: 19401469
- PMCID: PMC2742807
- DOI: 10.1074/jbc.R800083200
ENaC at the cutting edge: regulation of epithelial sodium channels by proteases
Abstract
Epithelial Na+ channels facilitate the transport of Na+ across high resistance epithelia. Proteolytic cleavage has an important role in regulating the activity of these channels by increasing their open probability. Specific proteases have been shown to activate epithelial Na+ channels by cleaving channel subunits at defined sites within their extracellular domains. This minireview addresses the mechanisms by which proteases activate this channel and the question of why proteolysis has evolved as a mechanism of channel activation.
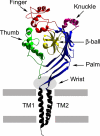
Figures

Similar articles
-
ENaC regulation by proteases and shear stress.Curr Mol Pharmacol. 2013 Mar;6(1):28-34. doi: 10.2174/18744672112059990027. Curr Mol Pharmacol. 2013. PMID: 23547932 Free PMC article. Review.
-
Cleavage in the {gamma}-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels.J Physiol. 2008 Oct 1;586(19):4587-608. doi: 10.1113/jphysiol.2008.154435. Epub 2008 Jul 31. J Physiol. 2008. PMID: 18669538 Free PMC article.
-
ENaC proteolytic regulation by channel-activating protease 2.J Gen Physiol. 2008 Nov;132(5):521-35. doi: 10.1085/jgp.200810030. Epub 2008 Oct 13. J Gen Physiol. 2008. PMID: 18852303 Free PMC article.
-
Intracellular Na+ regulates epithelial Na+ channel maturation.J Biol Chem. 2015 May 1;290(18):11569-77. doi: 10.1074/jbc.M115.640763. Epub 2015 Mar 12. J Biol Chem. 2015. PMID: 25767115 Free PMC article.
-
ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters.FEBS J. 2017 Feb;284(4):525-545. doi: 10.1111/febs.13840. Epub 2016 Sep 15. FEBS J. 2017. PMID: 27580245 Review.
Cited by
-
Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors.Exp Cell Res. 2012 May 15;318(9):1011-9. doi: 10.1016/j.yexcr.2012.02.024. Epub 2012 Mar 3. Exp Cell Res. 2012. PMID: 22405998 Free PMC article. Review.
-
Molecular principles of assembly, activation, and inhibition in epithelial sodium channel.Elife. 2020 Jul 30;9:e59038. doi: 10.7554/eLife.59038. Elife. 2020. PMID: 32729833 Free PMC article.
-
Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19.Eur J Clin Pharmacol. 2021 Sep;77(9):1275-1293. doi: 10.1007/s00228-021-03102-3. Epub 2021 Mar 27. Eur J Clin Pharmacol. 2021. PMID: 33772626 Free PMC article. Review.
-
Kidney ion handling genes and their interaction in blood pressure control.Biosci Rep. 2022 Nov 30;42(11):BSR20220977. doi: 10.1042/BSR20220977. Biosci Rep. 2022. PMID: 36305246 Free PMC article. Review.
-
Structural mechanisms underlying the function of epithelial sodium channel/acid-sensing ion channel.Curr Opin Nephrol Hypertens. 2011 Sep;20(5):555-60. doi: 10.1097/MNH.0b013e328348bcac. Curr Opin Nephrol Hypertens. 2011. PMID: 21709553 Free PMC article. Review.
References
-
- Snyder P. M. (2005) Endocrinology 146, 5079–5085 - PubMed
-
- Hughey R. P., Carattino M. D., Kleyman T. R. (2007) Curr. Opin. Nephrol. Hypertens. 16, 444–450 - PubMed
-
- Orce G. G., Castillo G. A., Margolius H. S. (1980) Am. J. Physiol. Renal Physiol. 239, F459–F465 - PubMed
-
- Vallet V., Chraibi A., Gaeggeler H. P., Horisberger J. D., Rossier B. C. (1997) Nature 389, 607–610 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

