Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen
- PMID: 19395555
- PMCID: PMC2716100
- DOI: 10.1152/ajpheart.00186.2009
Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen
Abstract
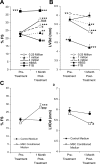
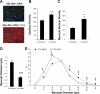
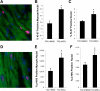
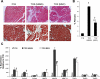
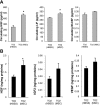
Heart failure carries a poor prognosis with few treatment options. While myocardial stem cell therapeutic trials have traditionally relied on intracoronary infusion or intramyocardial injection routes, these cell delivery methods are invasive and can introduce harmful scar tissue, arrhythmia, calcification, or microinfarction in the heart. Given that patients with heart failure are at an increased surgical risk, the development of a noninvasive stem cell therapeutic approach is logistically appealing. Taking advantage of the trophic effects of bone marrow mesenchymal stem cells (MSCs) and using a hamster heart failure model, the present study demonstrates a novel noninvasive therapeutic regimen via the direct delivery of MSCs into the skeletal muscle bed. Intramuscularly injected MSCs and MSC-conditioned medium each significantly improved ventricular function 1 mo after MSC administration. MSCs at 4 million cells/animal increased fractional shortening by approximately 40%, enhanced capillary and myocyte nuclear density by approximately 30% and approximately 80%, attenuated apoptosis by approximately 60%, and reduced fibrosis by approximately 50%. Myocyte regeneration was evidenced by an approximately twofold increase in the expression of cell cycle markers (Ki67 and phosphohistone H(3)) and an approximately 13% reduction in mean myocyte diameter. Increased circulating levels of hepatocyte growth factor (HGF), leukemia inhibitory factor, and macrophage colony-stimulating factor were associated with the mobilization of c-Kit-positive, CD31-positive, and CD133-positive progenitor cells and a subsequent increase in myocardial c-Kit-positive cells. Trophic effects of MSCs further activated the expression of HGF, IGF-II, and VEGF in the myocardium. The work highlights a cardiac repair mechanism mediated by trophic cross-talks among the injected MSCs, bone marrow, and heart that can be explored for noninvasive stem cell therapy.
Figures
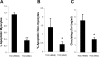
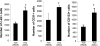
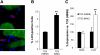
Similar articles
-
Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair.Biochem Biophys Res Commun. 2009 Dec 18;390(3):834-8. doi: 10.1016/j.bbrc.2009.10.058. Epub 2009 Oct 15. Biochem Biophys Res Commun. 2009. PMID: 19836359 Free PMC article.
-
Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2422-32. doi: 10.1152/ajpheart.00343.2011. Epub 2011 Sep 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21963833 Free PMC article.
-
Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells.Am J Physiol Regul Integr Comp Physiol. 2009 Nov;297(5):R1503-15. doi: 10.1152/ajpregu.00227.2009. Epub 2009 Sep 16. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19759338 Free PMC article.
-
Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy.Stem Cell Res Ther. 2021 Jun 23;12(1):361. doi: 10.1186/s13287-021-02443-1. Stem Cell Res Ther. 2021. PMID: 34162424 Free PMC article. Review.
-
Mesenchymal stem cells: future source for reparative medicine.Congest Heart Fail. 2005 Mar-Apr;11(2):87-91; quiz 92-3. doi: 10.1111/j.1527-5299.2005.03618.x. Congest Heart Fail. 2005. PMID: 15860974 Review.
Cited by
-
In vitro labeling of endothelial progenitor cells isolated from peripheral blood with superparamagnetic iron oxide nanoparticles.Mol Med Rep. 2012 Aug;6(2):282-6. doi: 10.3892/mmr.2012.912. Epub 2012 May 10. Mol Med Rep. 2012. PMID: 22580964 Free PMC article.
-
Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease.Cell Stem Cell. 2012 Mar 2;10(3):244-58. doi: 10.1016/j.stem.2012.02.005. Cell Stem Cell. 2012. PMID: 22385653 Free PMC article. Review.
-
sFRP2 activates Wnt/β-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling.Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C710-C719. doi: 10.1152/ajpcell.00137.2016. Epub 2016 Sep 7. Am J Physiol Cell Physiol. 2016. PMID: 27605451 Free PMC article.
-
Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14022-7. doi: 10.1073/pnas.0903201106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666564 Free PMC article.
-
Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine.Circ J. 2009 Oct;73(10):1774-82. doi: 10.1253/circj.cj-09-0588. Epub 2009 Sep 4. Circ J. 2009. PMID: 19729858 Free PMC article. Review.
References
-
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89: 1235–1249, 2003. - PubMed
-
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108: 863–868, 2003. - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068–14073, 2007. - PMC - PubMed
-
- Brann WM, Bennett LE, Keck BM, Hosenpud JD. Morbidity, functional status, and immunosuppressive therapy after heart transplantation: an analysis of the joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. J Heart Lung Transplant 17: 374–382, 1998. - PubMed
-
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

