Combinatorial morphogenesis of dendritic spines and filopodia by SPAR and alpha-actinin2
- PMID: 19393616
- PMCID: PMC2707853
- DOI: 10.1016/j.bbrc.2009.04.069
Combinatorial morphogenesis of dendritic spines and filopodia by SPAR and alpha-actinin2
Abstract
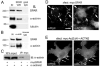
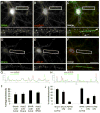
Rap small GTPases regulate excitatory synaptic strength and morphological plasticity of dendritic spines. Changes in spine structure are mediated by the F-actin cytoskeleton, but the link between Rap activity and actin dynamics is unclear. Here, we report a novel interaction between SPAR, a postsynaptic inhibitor of Rap, and alpha-actinin, a family of actin-cross-linking proteins. SPAR and alpha-actinin engage in bidirectional structural plasticity of dendritic spines: SPAR promotes spine head enlargement, whereas increased alpha-actinin2 expression favors dendritic spine elongation and thinning. Surprisingly, SPAR and alpha-actinin2 can function in an additive rather than antagonistic fashion at the same dendritic spine, generating combination spine/filopodia hybrids. These data identify a molecular pathway bridging the actin cytoskeleton and Rap at synapses, and suggest that formation of spines and filopodia are not necessarily opposing forms of structural plasticity.
Figures


Similar articles
-
Postsynaptic PDLIM5/Enigma Homolog binds SPAR and causes dendritic spine shrinkage.Mol Cell Neurosci. 2010 Feb;43(2):188-200. doi: 10.1016/j.mcn.2009.10.009. Epub 2009 Nov 10. Mol Cell Neurosci. 2010. PMID: 19900557 Free PMC article.
-
SNX26, a GTPase-activating protein for Cdc42, interacts with PSD-95 protein and is involved in activity-dependent dendritic spine formation in mature neurons.J Biol Chem. 2013 Oct 11;288(41):29453-66. doi: 10.1074/jbc.M113.468801. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003235 Free PMC article.
-
Actinin-4 Governs Dendritic Spine Dynamics and Promotes Their Remodeling by Metabotropic Glutamate Receptors.J Biol Chem. 2015 Jun 26;290(26):15909-20. doi: 10.1074/jbc.M115.640136. Epub 2015 May 5. J Biol Chem. 2015. PMID: 25944910 Free PMC article.
-
Role of actin cytoskeleton in dendritic spine morphogenesis.Neurochem Int. 2007 Jul-Sep;51(2-4):92-104. doi: 10.1016/j.neuint.2007.04.029. Epub 2007 May 13. Neurochem Int. 2007. PMID: 17590478 Review.
-
Emerging Roles of Filopodia and Dendritic Spines in Motoneuron Plasticity during Development and Disease.Neural Plast. 2016;2016:3423267. doi: 10.1155/2016/3423267. Epub 2015 Dec 30. Neural Plast. 2016. PMID: 26843990 Free PMC article. Review.
Cited by
-
ICAM-5 affects spine maturation by regulation of NMDA receptor binding to α-actinin.Biol Open. 2015 Jan 8;4(2):125-36. doi: 10.1242/bio.201410439. Biol Open. 2015. PMID: 25572420 Free PMC article.
-
Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization.J Cell Biol. 2016 Feb 15;212(4):449-63. doi: 10.1083/jcb.201509023. J Cell Biol. 2016. PMID: 26880202 Free PMC article.
-
Actin and Actin-Binding Proteins: Masters of Dendritic Spine Formation, Morphology, and Function.Open Neurosci J. 2009 Jan 1;3:54-66. doi: 10.2174/1874082000903020054. Open Neurosci J. 2009. PMID: 20717495 Free PMC article.
-
Postsynaptic PDLIM5/Enigma Homolog binds SPAR and causes dendritic spine shrinkage.Mol Cell Neurosci. 2010 Feb;43(2):188-200. doi: 10.1016/j.mcn.2009.10.009. Epub 2009 Nov 10. Mol Cell Neurosci. 2010. PMID: 19900557 Free PMC article.
-
The TRIM9/TRIM67 neuronal interactome reveals novel activators of morphogenesis.Mol Biol Cell. 2021 Feb 15;32(4):314-330. doi: 10.1091/mbc.E20-10-0622. Epub 2020 Dec 30. Mol Biol Cell. 2021. PMID: 33378226 Free PMC article.
References
-
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. - PubMed
-
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
-
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

