Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6
- PMID: 19386608
- PMCID: PMC2719412
- DOI: 10.1074/jbc.M808506200
Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6
Abstract
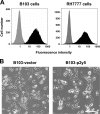
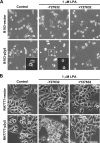
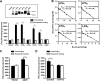
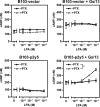
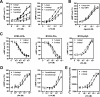
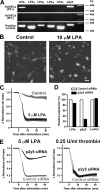
p2y5 is an orphan G protein-coupled receptor that is closely related to the fourth lysophosphatidic acid (LPA) receptor, LPA4. Here we report that p2y5 is a novel LPA receptor coupling to the G13-Rho signaling pathway. "LPA receptor-null" RH7777 and B103 cells exogenously expressing p2y5 showed [3H]LPA binding, LPA-induced [35S]guanosine 5'-3-O-(thio)triphosphate binding, Rho-dependent alternation of cellular morphology, and Gs/13 chimeric protein-mediated cAMP accumulation. LPA-induced contraction of human umbilical vein endothelial cells was suppressed by small interfering RNA knockdown of endogenously expressed p2y5. We also found that 2-acyl-LPA had higher activity to p2y5 than 1-acyl-LPA. A recent study has suggested that p2y5 is an LPA receptor essential for human hair growth. We confirmed that p2y5 is a functional LPA receptor and propose to designate this receptor LPA6.
Figures
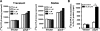
Similar articles
-
P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion.Am J Physiol Gastrointest Liver Physiol. 2009 Oct;297(4):G641-54. doi: 10.1152/ajpgi.00191.2009. Epub 2009 Aug 13. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19679818 Free PMC article.
-
LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation.J Biol Chem. 2007 Feb 16;282(7):4310-4317. doi: 10.1074/jbc.M610826200. Epub 2006 Dec 13. J Biol Chem. 2007. PMID: 17166850
-
LPA-producing enzyme PA-PLA₁α regulates hair follicle development by modulating EGFR signalling.EMBO J. 2011 Aug 19;30(20):4248-60. doi: 10.1038/emboj.2011.296. EMBO J. 2011. PMID: 21857648 Free PMC article.
-
Non-Edg family lysophosphatidic acid (LPA) receptors.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):57-65. doi: 10.1016/j.prostaglandins.2009.06.001. Epub 2009 Jun 12. Prostaglandins Other Lipid Mediat. 2009. PMID: 19524700 Review.
-
LPA(3), a unique G protein-coupled receptor for lysophosphatidic acid.Prog Lipid Res. 2010 Oct;49(4):335-42. doi: 10.1016/j.plipres.2010.03.001. Epub 2010 Mar 15. Prog Lipid Res. 2010. PMID: 20230855 Review.
Cited by
-
Targeting Lysophosphatidic Acid in Cancer: The Issues in Moving from Bench to Bedside.Cancers (Basel). 2019 Oct 10;11(10):1523. doi: 10.3390/cancers11101523. Cancers (Basel). 2019. PMID: 31658655 Free PMC article. Review.
-
Aiming drug discovery at lysophosphatidic acid targets.Br J Pharmacol. 2010 Sep;161(2):241-70. doi: 10.1111/j.1476-5381.2010.00815.x. Br J Pharmacol. 2010. PMID: 20735414 Free PMC article. Review.
-
Chemical Proteomic Profiling of Lysophosphatidic Acid-Binding Proteins.Anal Chem. 2019 Dec 17;91(24):15365-15369. doi: 10.1021/acs.analchem.9b04850. Epub 2019 Nov 27. Anal Chem. 2019. PMID: 31765128 Free PMC article.
-
Insights into the pharmacological relevance of lysophospholipid receptors.Br J Pharmacol. 2012 Feb;165(4):829-44. doi: 10.1111/j.1476-5381.2011.01622.x. Br J Pharmacol. 2012. PMID: 21838759 Free PMC article. Review.
-
Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy.Biochem Biophys Res Commun. 2018 Jul 2;501(4):1048-1054. doi: 10.1016/j.bbrc.2018.05.106. Epub 2018 May 24. Biochem Biophys Res Commun. 2018. PMID: 29778535 Free PMC article.
References
-
- Tokumura A. ( 1995) Prog. Lipid Res. 34, 151– 184 - PubMed
-
- Ishii I., Fukushima N., Ye X., Chun J. ( 2004) Annu. Rev. Biochem. 73, 321– 354 - PubMed
-
- Herr D. R., Chun J. ( 2007) Curr. Drug Targets 8, 155– 167 - PubMed
-
- Murph M., Tanaka T., Liu S., Mills G. B. ( 2006) Clin. Cancer Res. 12, 6598– 6602 - PubMed
-
- Watterson K. R., Lanning D. A., Diegelmann R. F., Spiegel S. ( 2007) Wound Repair Regen. 15, 607– 616 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

