The Xpc gene markedly affects cell survival in mouse bone marrow
- PMID: 19372135
- PMCID: PMC2701989
- DOI: 10.1093/mutage/gep011
The Xpc gene markedly affects cell survival in mouse bone marrow
Abstract
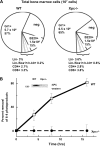
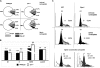
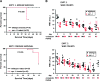
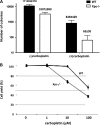
The XPC protein (encoded by the xeroderma pigmentosum Xpc gene) is a key DNA damage recognition factor that is required for global genomic nucleotide excision repair (G-NER). In contrast to transcription-coupled nucleotide excision repair (TC-NER), XPC and G-NER have been reported to contribute only modestly to cell survival after DNA damage. Previous studies were conducted using fibroblasts of human or mouse origin. Since the advent of Xpc-/- mice, no study has focused on the bone marrow of these mice. We used carboplatin to induce DNA damage in Xpc-/- and strain-matched wild-type mice. Using several independent methods, Xpc-/- bone marrow was approximately 10-fold more sensitive to carboplatin than the wild type. Importantly, 12/20 Xpc-/- mice died while 0/20 wild-type mice died. We conclude that G-NER, and XPC specifically, can contribute substantially to cell survival. The data are important in the context of cancer chemotherapy, where Xpc gene status and G-NER may be determinants of response to DNA-damaging agents including carboplatin. Additionally, altered cell cycles and altered DNA damage signalling may contribute to the cell survival end point.
Figures


Similar articles
-
Slow accumulation of mutations in Xpc-/- mice upon induction of oxidative stress.DNA Repair (Amst). 2013 Dec;12(12):1081-6. doi: 10.1016/j.dnarep.2013.08.019. Epub 2013 Sep 29. DNA Repair (Amst). 2013. PMID: 24084170 Free PMC article.
-
The role of XPC: implications in cancer and oxidative DNA damage.Mutat Res. 2011 Nov-Dec;728(3):107-17. doi: 10.1016/j.mrrev.2011.07.001. Epub 2011 Jul 7. Mutat Res. 2011. PMID: 21763452 Free PMC article.
-
Cell-type-specific consequences of nucleotide excision repair deficiencies: Embryonic stem cells versus fibroblasts.DNA Repair (Amst). 2008 Oct 1;7(10):1659-69. doi: 10.1016/j.dnarep.2008.06.009. Epub 2008 Jul 26. DNA Repair (Amst). 2008. PMID: 18634906
-
XPC multifaceted roles beyond DNA damage repair: p53-dependent and p53-independent functions of XPC in cell fate decisions.Mutat Res Rev Mutat Res. 2022 Jan-Jun;789:108400. doi: 10.1016/j.mrrev.2021.108400. Epub 2021 Nov 20. Mutat Res Rev Mutat Res. 2022. PMID: 35690409 Review.
-
Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair.DNA Repair (Amst). 2016 Aug;44:110-117. doi: 10.1016/j.dnarep.2016.05.015. Epub 2016 May 20. DNA Repair (Amst). 2016. PMID: 27264556 Review.
Cited by
-
Xeroderma Pigmentosum Complementation Group C (XPC): Emerging Roles in Non-Dermatologic Malignancies.Front Oncol. 2022 Apr 21;12:846965. doi: 10.3389/fonc.2022.846965. eCollection 2022. Front Oncol. 2022. PMID: 35530314 Free PMC article. Review.
-
Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells.Int J Mol Sci. 2015 Jan 22;16(2):2366-85. doi: 10.3390/ijms16022366. Int J Mol Sci. 2015. PMID: 25622253 Free PMC article. Review.
-
Genomic and functional integrity of the hematopoietic system requires tolerance of oxidative DNA lesions.Blood. 2017 Sep 28;130(13):1523-1534. doi: 10.1182/blood-2017-01-764274. Epub 2017 Aug 21. Blood. 2017. PMID: 28827409 Free PMC article.
-
Seleno-L-Methionine Modulation of Nucleotide Excision DNA Repair Relevant to Cancer Prevention and Chemotherapy.Mol Cell Pharmacol. 2009;1(4):218-221. Mol Cell Pharmacol. 2009. PMID: 20336178 Free PMC article.
-
The "Two faces" of Tumor Suppressor p53-revisited.Mol Cell Pharmacol. 2010 Jan 1;2(3):117-119. Mol Cell Pharmacol. 2010. PMID: 20686673 Free PMC article.
References
-
- Friedberg EC, Aguilera AM, Gellert M, et al. DNA repair, from molecular mechanism to human disease. DNA Repair (Amst.) 2006;5:986–996. - PubMed
-
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. - PubMed
-
- Berg RJ, Ruven HJ, Sands AT, deGruiji FR, Mullenders LH. Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J. Invest. Dermatol. 1998;110:405–409. - PubMed
-
- Wolf T, Densmore JJ. Pegfilgrastim use during chemotherapy: current and future applications. Curr. Hematol. Rep. 2004;3:419–423. - PubMed

