Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis
- PMID: 19369650
- PMCID: PMC2849825
- DOI: 10.1095/biolreprod.108.075200
Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis
Abstract
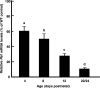
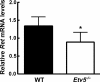
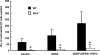
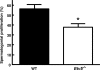
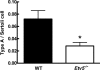
Mice that are ets variant gene 5 (ETV5) null (Etv5(-/-)) undergo the first wave of spermatogenesis but lose all spermatogonial stem cells (SSCs) during this time. The SSC loss in Etv5(-/-) mice begins during the neonatal period, suggesting a role for ETV5 in SSC self-renewal during this period. Herein, we show that Etv5 mRNA was present in perinatal mouse testis and that ETV5 was expressed in fetal Sertoli cells and by germ cells and Sertoli cells during the neonatal period. Transplantation of Etv5(-/-) germ cells failed to establish spermatogenesis in W/W(v) mice testes, indicating that germ cell ETV5 has a key role in establishment or self-renewal of transplanted SSCs. The SSC self-renewal is stimulated by glial cell-derived neurotrophic factor (GDNF) acting through the RET/GDNF family receptor alpha 1 (GFRA1) receptor complex in SSCs. Immunohistochemistry, quantitative PCR, and laser capture microdissection revealed decreased RET mRNA and protein expression in spermatogonia of neonatal Etv5(-/-) mice by Postnatal Days 4-8, indicating that disrupted GDNF/RET/GFRA1 signaling may occur before initial spermatogonial stem/progenitor cell decrease. Etv5(-/-) spermatogonia had reduced proliferation in vivo and in vitro. Decreased cell proliferation may cause the observed decreases in the number of type A spermatogonia (Postnatal Day 17) and daily sperm production (Postnatal Day 30) in Etv5(-/-) mice, indicating quantitative impairments in the first wave of spermatogenesis. In conclusion, ETV5 is expressed beginning in fetal Sertoli cells and can potentially have effects on neonatal Sertoli cells and germ cells. In addition, ETV5 has critical effects on neonatal spermatogonial proliferation, which may involve impaired signaling through the RET receptor.
Figures




Similar articles
-
Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal.Stem Cell Reports. 2015 Mar 10;4(3):489-502. doi: 10.1016/j.stemcr.2015.01.010. Epub 2015 Feb 12. Stem Cell Reports. 2015. PMID: 25684228 Free PMC article.
-
Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate.Biol Reprod. 2006 Feb;74(2):314-21. doi: 10.1095/biolreprod.105.047365. Epub 2005 Oct 19. Biol Reprod. 2006. PMID: 16237148
-
The production of glial cell line-derived neurotrophic factor by human sertoli cells is substantially reduced in sertoli cell-only testes.Hum Reprod. 2017 May 1;32(5):1108-1117. doi: 10.1093/humrep/dex061. Hum Reprod. 2017. PMID: 28369535 Free PMC article.
-
ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood testes barrier function and testicular immune privilege.Ann N Y Acad Sci. 2007 Dec;1120:144-51. doi: 10.1196/annals.1411.005. Epub 2007 Oct 2. Ann N Y Acad Sci. 2007. PMID: 17911411 Free PMC article. Review.
-
Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling.Reproduction. 2015 Apr;149(4):R159-67. doi: 10.1530/REP-14-0481. Epub 2014 Dec 12. Reproduction. 2015. PMID: 25504872 Review.
Cited by
-
The Transcription Factor ETV5 Mediates BRAFV600E-Induced Proliferation and TWIST1 Expression in Papillary Thyroid Cancer Cells.Neoplasia. 2018 Nov;20(11):1121-1134. doi: 10.1016/j.neo.2018.09.003. Epub 2018 Sep 25. Neoplasia. 2018. PMID: 30265861 Free PMC article.
-
Spermatogonial stem cells, infertility and testicular cancer.J Cell Mol Med. 2011 Mar;15(3):468-83. doi: 10.1111/j.1582-4934.2010.01242.x. J Cell Mol Med. 2011. PMID: 21155977 Free PMC article. Review.
-
The mTORC1 component RPTOR is required for maintenance of the foundational spermatogonial stem cell pool in mice†.Biol Reprod. 2019 Feb 1;100(2):429-439. doi: 10.1093/biolre/ioy198. Biol Reprod. 2019. PMID: 30202948 Free PMC article.
-
MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells.Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12740-5. doi: 10.1073/pnas.1109987108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768389 Free PMC article.
-
Either Kras activation or Pten loss similarly enhance the dominant-stable CTNNB1-induced genetic program to promote granulosa cell tumor development in the ovary and testis.Oncogene. 2012 Mar 22;31(12):1504-20. doi: 10.1038/onc.2011.341. Epub 2011 Aug 22. Oncogene. 2012. PMID: 21860425 Free PMC article.
References
-
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G.Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 2006; 440: 1199–1203. - PubMed
-
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 2004; 119: 1001–1012. - PubMed
-
- Griswold MD.The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 1998; 9: 411–416. - PubMed
-
- Ogawa T, Ohmura M, Ohbo K.The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol 2005; 82: 381–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

