Uncoupling stress granule assembly and translation initiation inhibition
- PMID: 19369421
- PMCID: PMC2688547
- DOI: 10.1091/mbc.e08-10-1061
Uncoupling stress granule assembly and translation initiation inhibition
Abstract
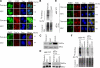
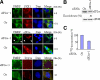
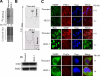
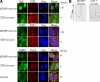
Cytoplasmic stress granules (SGs) are specialized regulatory sites of mRNA translation that form under different stress conditions known to inhibit translation initiation. The formation of SG occurs via two pathways; the eukaryotic initiation factor (eIF) 2alpha phosphorylation-dependent pathway mediated by stress and the eIF2alpha phosphorylation-independent pathway mediated by inactivation of the translation initiation factors eIF4A and eIF4G. In this study, we investigated the effects of targeting different translation initiation factors and steps in SG formation in HeLa cells. By depleting eIF2alpha, we demonstrate that reduced levels of the eIF2.GTP.Met-tRNAi(Met) ternary translation initiation complexes is sufficient to induce SGs. Likewise, reduced levels of eIF4B, eIF4H, or polyA-binding protein, also trigger SG formation. In contrast, depletion of the cap-binding protein eIF4E or preventing its assembly into eIF4F results in modest SG formation. Intriguingly, interfering with the last step of translation initiation by blocking the recruitment of 60S ribosome either with 2-(4-methyl-2,6-dinitroanilino)-N-methylpropionamideis or through depletion of the large ribosomal subunits protein L28 does not induce SG assembly. Our study identifies translation initiation steps and factors involved in SG formation as well as those that can be targeted without induction of SGs.
Figures



Similar articles
-
Stress Granule Induction after Brain Ischemia Is Independent of Eukaryotic Translation Initiation Factor (eIF) 2α Phosphorylation and Is Correlated with a Decrease in eIF4B and eIF4E Proteins.J Biol Chem. 2016 Dec 30;291(53):27252-27264. doi: 10.1074/jbc.M116.738989. Epub 2016 Nov 11. J Biol Chem. 2016. PMID: 27836976 Free PMC article.
-
Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules.Mol Biol Cell. 2002 Jan;13(1):195-210. doi: 10.1091/mbc.01-05-0221. Mol Biol Cell. 2002. PMID: 11809833 Free PMC article.
-
Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation.Mol Biol Cell. 2006 Oct;17(10):4212-9. doi: 10.1091/mbc.e06-04-0318. Epub 2006 Jul 26. Mol Biol Cell. 2006. PMID: 16870703 Free PMC article.
-
Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
-
Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation.Cell Stress Chaperones. 2002 Apr;7(2):213-21. doi: 10.1379/1466-1268(2002)007<0213:vstroe>2.0.co;2. Cell Stress Chaperones. 2002. PMID: 12380690 Free PMC article. Review.
Cited by
-
Targeting Nup358/RanBP2 by a viral protein disrupts stress granule formation.PLoS Pathog. 2022 Dec 1;18(12):e1010598. doi: 10.1371/journal.ppat.1010598. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36455064 Free PMC article.
-
TDP-43 aggregation in neurodegeneration: are stress granules the key?Brain Res. 2012 Jun 26;1462:16-25. doi: 10.1016/j.brainres.2012.02.032. Epub 2012 Feb 22. Brain Res. 2012. PMID: 22405725 Free PMC article. Review.
-
Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules.BMC Mol Biol. 2016 Aug 30;17(1):21. doi: 10.1186/s12867-016-0072-x. BMC Mol Biol. 2016. PMID: 27578149 Free PMC article.
-
Repeat-associated non-AUG (RAN) translation mechanisms are running into focus for GGGGCC-repeat associated ALS/FTD.Prog Neurobiol. 2019 Dec;183:101697. doi: 10.1016/j.pneurobio.2019.101697. Epub 2019 Sep 21. Prog Neurobiol. 2019. PMID: 31550516 Free PMC article. Review.
-
CoCl2-mimicked Hypoxia Induces the Assembly of Stress Granules in Trophoblast Cells Via eIF2α Phosphorylation-dependent and - Independent Pathways.Curr Mol Med. 2024;24(10):1291-1300. doi: 10.2174/1566524023666230913111300. Curr Mol Med. 2024. PMID: 37711098
References
-
- Anderson P., Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 2008;33:141–150. - PubMed
-
- Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008;10:1324–1332. - PubMed
-
- Baxter R., Knell V. C., Somerville H. J., Swain H. M., Weeks D. P. Effect of MDMP on protein synthesis in wheat and bacteria. Nat. New Biol. 1973;243:139–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

