Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene
- PMID: 19365082
- PMCID: PMC2700315
- DOI: 10.1182/blood-2008-10-186684
Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene
Abstract
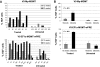
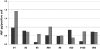
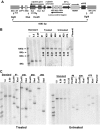
Correction of murine models of beta-thalassemia has been achieved through high-level globin lentiviral vector gene transfer into mouse hematopoietic stem cells (HSCs). However, transduction of human HSCs is less robust and may be inadequate to achieve therapeutic levels of genetically modified erythroid cells. We therefore developed a double gene lentiviral vector encoding both human gamma-globin under the transcriptional control of erythroid regulatory elements and methylguanine methyltransferase (MGMT), driven by a constitutive cellular promoter. MGMT expression provides cellular resistance to alkylator drugs, which can be administered to kill residual untransduced, diseased HSCs, whereas transduced cells are protected. Mice transplanted with beta-thalassemic HSCs transduced with a gamma-globin/MGMT vector initially had subtherapeutic levels of red cells expressing gamma-globin. To enrich gamma-globin-expressing cells, transplanted mice were treated with the alkylator agent 1,3-bis-chloroethyl-1-nitrosourea. This resulted in significant increases in the number of gamma-globin-expressing red cells and the amount of fetal hemoglobin, leading to resolution of anemia. Selection of transduced HSCs was also obtained when cells were drug-treated before transplantation. Mice that received these cells demonstrated reconstitution with therapeutic levels of gamma-globin-expressing cells. These data suggest that MGMT-based drug selection holds promise as a modality to improve gene therapy for beta-thalassemia.
Figures
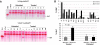

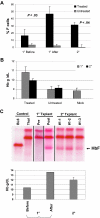
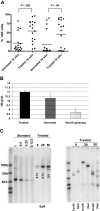
Similar articles
-
Lentiviral Transfer of γ-Globin with Fusion Gene NUP98-HOXA10HD Expands Hematopoietic Stem Cells and Ameliorates Murine β-Thalassemia.Mol Ther. 2017 Mar 1;25(3):593-605. doi: 10.1016/j.ymthe.2017.01.019. Epub 2017 Feb 9. Mol Ther. 2017. PMID: 28190779 Free PMC article.
-
Successful treatment of murine beta-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells.Blood. 2003 Jul 15;102(2):506-13. doi: 10.1182/blood-2003-03-0677. Epub 2003 Mar 27. Blood. 2003. PMID: 12663444
-
The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number.Blood. 2003 Mar 15;101(6):2175-83. doi: 10.1182/blood-2002-07-2211. Epub 2002 Oct 31. Blood. 2003. PMID: 12411297
-
Successful correction of the human Cooley's anemia beta-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator.Ann N Y Acad Sci. 2005;1054:238-49. doi: 10.1196/annals.1345.030. Ann N Y Acad Sci. 2005. PMID: 16339671 Review.
-
Hematopoietic stem cell gene transfer for the treatment of hemoglobin disorders.Hematology Am Soc Hematol Educ Program. 2009:690-7. doi: 10.1182/asheducation-2009.1.690. Hematology Am Soc Hematol Educ Program. 2009. PMID: 20008255 Review.
Cited by
-
Cell Membrane-associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells.Mol Ther. 2012 Jun;20(6):1158-66. doi: 10.1038/mt.2012.41. Epub 2012 Mar 20. Mol Ther. 2012. PMID: 22434139 Free PMC article.
-
A zinc-finger transcriptional activator designed to interact with the gamma-globin gene promoters enhances fetal hemoglobin production in primary human adult erythroblasts.Blood. 2010 Apr 15;115(15):3033-41. doi: 10.1182/blood-2009-08-240556. Epub 2010 Feb 26. Blood. 2010. PMID: 20190190 Free PMC article.
-
Chemoprotection in brain tumor patients: another success for stem cell gene therapy.Mol Ther. 2012 Aug;20(8):1485-7. doi: 10.1038/mt.2012.139. Mol Ther. 2012. PMID: 22850719 Free PMC article. No abstract available.
-
Repair of O4-alkylthymine by O6-alkylguanine-DNA alkyltransferases.J Biol Chem. 2010 Mar 12;285(11):8185-95. doi: 10.1074/jbc.M109.045518. Epub 2009 Dec 21. J Biol Chem. 2010. PMID: 20026607 Free PMC article.
-
Lentiviral Transfer of γ-Globin with Fusion Gene NUP98-HOXA10HD Expands Hematopoietic Stem Cells and Ameliorates Murine β-Thalassemia.Mol Ther. 2017 Mar 1;25(3):593-605. doi: 10.1016/j.ymthe.2017.01.019. Epub 2017 Feb 9. Mol Ther. 2017. PMID: 28190779 Free PMC article.
References
-
- Nienhuis AW. Development of gene therapy for blood disorders. Blood. 2008;111:4431–4444. - PubMed
-
- May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. - PubMed
-
- Pawliuk R, Westerman KA, Fabry ME, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. - PubMed
-
- Persons DA, Hargrove PW, Allay ER, Hanawa H, Nienhuis AW. The degree of phenotypic correction of murine beta-thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

