Formation of Hirano bodies after inducible expression of a modified form of an actin-cross-linking protein
- PMID: 19363062
- PMCID: PMC2698299
- DOI: 10.1128/EC.00379-08
Formation of Hirano bodies after inducible expression of a modified form of an actin-cross-linking protein
Abstract
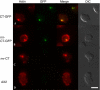
Hirano bodies are cytoplasmic inclusions composed mainly of actin and actin-associated proteins. The formation of Hirano bodies during various neurodegenerative disorders, including Alzheimer's disease and amyotrophic lateral sclerosis, has been reported. Although the underlying molecular mechanisms that lead to the formation of these inclusions in the brain are not known, expression of the C-terminal fragment (CT) (amino acids 124 to 295) from the endogenous 34-kDa actin-binding protein of Dictyostelium discoideum leads to the formation of actin inclusions in vivo. In the current study, we report the development of an inducible expression system to study the early phases of Hirano body formation using an inducible promoter system (rnrB). By fusing the CT to a green fluorescent protein (CT-GFP), we monitored protein expression and localization by fluorescence microscopy, flow cytometry, and Western blot analysis. We observed an increase in the number and size of inclusions formed following induction of the CT-GFP vector system. Time-lapse microscopy studies revealed that the CT-GFP foci associated with the cell cortex and fused to form a single large aggregate. Transmission electron microscopy further demonstrates that these inclusions have a highly ordered ultrastructure, a pathological hallmark of Hirano bodies observed in postmortem brain samples from patients with various neurodegenerative disorders. Collectively, this system provides a method to visualize and characterize the events that surround early actin inclusion formation in a eukaryotic model.
Figures

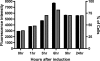

Similar articles
-
Formation of Hirano bodies in Dictyostelium and mammalian cells induced by expression of a modified form of an actin-crosslinking protein.J Cell Sci. 2002 May 1;115(Pt 9):1939-49. doi: 10.1242/jcs.115.9.1939. J Cell Sci. 2002. PMID: 11956325
-
Formation of Hirano bodies induced by expression of an actin cross-linking protein with a gain-of-function mutation.Eukaryot Cell. 2003 Aug;2(4):778-87. doi: 10.1128/EC.2.4.778-787.2003. Eukaryot Cell. 2003. PMID: 12912897 Free PMC article.
-
A cell culture model for investigation of Hirano bodies.Acta Neuropathol. 2008 Feb;115(2):205-17. doi: 10.1007/s00401-007-0275-9. Epub 2007 Nov 3. Acta Neuropathol. 2008. PMID: 17978823
-
Hirano bodies and Alzheimer's disease.Kaohsiung J Med Sci. 1997 Jan;13(1):10-8. Kaohsiung J Med Sci. 1997. PMID: 9130818 Review.
-
The complexity and diversity of the actin cytoskeleton of trypanosomatids.Mol Biochem Parasitol. 2020 May;237:111278. doi: 10.1016/j.molbiopara.2020.111278. Epub 2020 Apr 28. Mol Biochem Parasitol. 2020. PMID: 32353561 Review.
Cited by
-
Requirements for Hirano body formation.Eukaryot Cell. 2014 May;13(5):625-34. doi: 10.1128/EC.00044-14. Epub 2014 Mar 14. Eukaryot Cell. 2014. PMID: 24632241 Free PMC article.
-
De novo actin polymerization is required for model Hirano body formation in Dictyostelium.Biol Open. 2016 Jun 15;5(6):807-18. doi: 10.1242/bio.014944. Biol Open. 2016. PMID: 27215322 Free PMC article.
-
Transgenic mouse model for the formation of Hirano bodies.BMC Neurosci. 2011 Oct 6;12:97. doi: 10.1186/1471-2202-12-97. BMC Neurosci. 2011. PMID: 21978358 Free PMC article.
-
Hirano body expression impairs spatial working memory in a novel mouse model.Acta Neuropathol Commun. 2014 Sep 2;2:131. doi: 10.1186/s40478-014-0131-9. Acta Neuropathol Commun. 2014. PMID: 25178488 Free PMC article.
References
-
- Davis, R. C., R. Furukawa, and M. Fechheimer. 2008. A cell culture model for investigation of Hirano bodies. Acta Neuropathol. 115205-217. - PubMed
-
- Fey, P., A. S. Kowal, P. Gaudet, K. E. Pilcher, and R. L. Chisholm. 2007. Protocols for growth and development of Dictyostelium discoideum. Nat. Protoc. 21307-1316. - PubMed
-
- Galloway, P. G., G. Perry, and P. Gambetti. 1987. Hirano body filaments contain actin and actin-associated proteins. J. Neuropathol. Exp. Neurol. 46185-199. - PubMed
-
- Gaudet, P., K. E. Pilcher, P. Fey, and R. L. Chisholm. 2007. Transformation of Dictyostelium discoideum with plasmid DNA. Nat. Protoc. 21317-1324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

