A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system
- PMID: 19360898
- PMCID: PMC2730994
- DOI: 10.1002/ana.21623
A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system
Abstract
Objective: Accumulation of amyloid-beta (Abeta) by overproduction or underclearance in the central nervous system (CNS) is hypothesized to be a necessary event in the pathogenesis of Alzheimer's disease. However, previously, there has not been a method to determine drug effects on Abeta production or clearance in the human CNS. The objective of this study was to determine the effects of a gamma-secretase inhibitor on the production of Abeta in the human CNS.
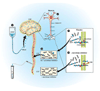
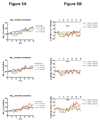
Methods: We utilized a recently developed method of stable-isotope labeling combined with cerebrospinal fluid sampling to directly measure Abeta production during treatment of a gamma-secretase inhibitor, LY450139. We assessed whether this drug could decrease CNS Abeta production in healthy men (age range, 21-50 years) at single oral doses of 100, 140, or 280mg (n = 5 per group).
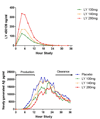
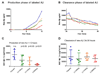
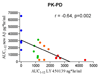
Results: LY450139 significantly decreased the production of CNS Abeta in a dose-dependent fashion, with inhibition of Abeta generation of 47, 52, and 84% over a 12-hour period with doses of 100, 140, and 280mg, respectively. There was no difference in Abeta clearance.
Interpretation: Stable isotope labeling of CNS proteins can be utilized to assess the effects of drugs on the production and clearance rates of proteins targeted as potential disease-modifying treatments for Alzheimer's disease and other CNS disorders. Results from this approach can assist in making decisions about drug dosing and frequency in the design of larger and longer clinical trials for diseases such as Alzheimer's disease, and may accelerate effective drug validation. Ann Neurol 2009.
Conflict of interest statement
Conflict of Interest Statement: Drs. Bateman and Holtzman are co-founders of a company (C2N Diagnostics) which has licensed a pending Washington University patent on the technology described in this article. Drs. Siemers, Friedrich, Demattos, May and Paul are employees of Eli Lilly, the sponsor of this study.
Figures
Comment in
-
In response to "a gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system".Ann Neurol. 2010 Jan;67(1):143-4; author reply 144-5. doi: 10.1002/ana.21929. Ann Neurol. 2010. PMID: 20186950 No abstract available.
Similar articles
-
Acute effect on the Aβ isoform pattern in CSF in response to γ-secretase modulator and inhibitor treatment in dogs.J Alzheimers Dis. 2010;21(3):1005-12. doi: 10.3233/JAD-2010-100573. J Alzheimers Dis. 2010. PMID: 20634579
-
Amyloid-β(1-15/16) as a marker for γ-secretase inhibition in Alzheimer's disease.J Alzheimers Dis. 2012;31(2):335-41. doi: 10.3233/JAD-2012-120508. J Alzheimers Dis. 2012. PMID: 22531418 Free PMC article. Clinical Trial.
-
Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers.Clin Neuropharmacol. 2005 May-Jun;28(3):126-32. doi: 10.1097/01.wnf.0000167360.27670.29. Clin Neuropharmacol. 2005. PMID: 15965311 Clinical Trial.
-
Advances in the identification of γ-secretase inhibitors for the treatment of Alzheimer's disease.Expert Opin Drug Discov. 2012 Jan;7(1):19-37. doi: 10.1517/17460441.2012.645534. Epub 2011 Dec 14. Expert Opin Drug Discov. 2012. PMID: 22468891 Review.
-
Evaluation of the performance of novel Aβ isoforms as theragnostic markers in Alzheimer's disease: from the cell to the patient.Neurodegener Dis. 2012;10(1-4):138-40. doi: 10.1159/000334537. Epub 2012 Feb 1. Neurodegener Dis. 2012. PMID: 22302034 Review.
Cited by
-
Design and synthesis of novel methoxypyridine-derived gamma-secretase modulators.Bioorg Med Chem. 2020 Nov 15;28(22):115734. doi: 10.1016/j.bmc.2020.115734. Epub 2020 Sep 1. Bioorg Med Chem. 2020. PMID: 33007551 Free PMC article.
-
In Vivo Characterization of a Novel γ-Secretase Inhibitor SCH 697466 in Rodents and Investigation of Strategies for Managing Notch-Related Side Effects.Int J Alzheimers Dis. 2013;2013:823528. doi: 10.1155/2013/823528. Epub 2013 Mar 14. Int J Alzheimers Dis. 2013. PMID: 23573456 Free PMC article.
-
Mining the secrets of the CSF: developing biomarkers of neurodegeneration.J Clin Invest. 2012 Sep;122(9):3051-3. doi: 10.1172/JCI65309. Epub 2012 Aug 27. J Clin Invest. 2012. PMID: 22922253 Free PMC article.
-
Alzheimer's disease pathology is attenuated in a CD38-deficient mouse model.Ann Neurol. 2015 Jul;78(1):88-103. doi: 10.1002/ana.24425. Epub 2015 May 25. Ann Neurol. 2015. PMID: 25893674 Free PMC article.
-
Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease?Nat Rev Neurosci. 2010 May;11(5):361-70. doi: 10.1038/nrn2808. Nat Rev Neurosci. 2010. PMID: 20216546 Free PMC article. Review.
References
-
- Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. - PubMed
-
- Skovronsky DM, Lee VMY, Trojanowski JQ. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annual Review of Pathology. 2006;Vol. 1:151–170. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Golde TE, Dickson D, Hutton M. Filling the gaps in the Aβ cascade hypothesis of Alzheimer's disease. Current Alzheimer Research. 2006;3:421–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 AG030946-02/AG/NIA NIH HHS/United States
- 1UL1 RR024992/RR/NCRR NIH HHS/United States
- P30 DK056341-08/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- 2P60 DK020579-31/DK/NIDDK NIH HHS/United States
- K23 AG030946/AG/NIA NIH HHS/United States
- SP30 DK056341-08/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- P41 RR000954/RR/NCRR NIH HHS/United States
- P30 DK056341-09/DK/NIDDK NIH HHS/United States
- 5P41 RR000954-32/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

