SLP-2 is required for stress-induced mitochondrial hyperfusion
- PMID: 19360003
- PMCID: PMC2693158
- DOI: 10.1038/emboj.2009.89
SLP-2 is required for stress-induced mitochondrial hyperfusion
Abstract
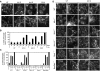
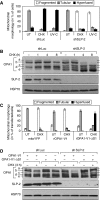
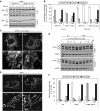
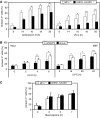
Mitochondria are dynamic organelles, the morphology of which results from an equilibrium between two opposing processes, fusion and fission. Mitochondrial fusion relies on dynamin-related GTPases, the mitofusins (MFN1 and 2) in the outer mitochondrial membrane and OPA1 (optic atrophy 1) in the inner mitochondrial membrane. Apart from a role in the maintenance of mitochondrial DNA, little is known about the physiological role of mitochondrial fusion. Here we report that mitochondria hyperfuse and form a highly interconnected network in cells exposed to selective stresses. This process precedes mitochondrial fission when it is triggered by apoptotic stimuli such as UV irradiation or actinomycin D. Stress-induced mitochondrial hyperfusion (SIMH) is independent of MFN2, BAX/BAK, and prohibitins, but requires L-OPA1, MFN1, and the mitochondrial inner membrane protein SLP-2. In the absence of SLP-2, L-OPA1 is lost and SIMH is prevented. SIMH is accompanied by increased mitochondrial ATP production and represents a novel adaptive pro-survival response against stress.
Figures



Comment in
-
Fussy mitochondria fuse in response to stress.EMBO J. 2009 Jun 3;28(11):1533-4. doi: 10.1038/emboj.2009.130. EMBO J. 2009. PMID: 19494844 Free PMC article. No abstract available.
Similar articles
-
Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion.Mol Biol Cell. 2009 Aug;20(15):3525-32. doi: 10.1091/mbc.e09-03-0252. Epub 2009 May 28. Mol Biol Cell. 2009. PMID: 19477917 Free PMC article.
-
[Advances in mitochondrial fusion-fission and Ca2+ signaling in mammals].Sheng Li Ke Xue Jin Zhan. 2010 Jun;41(3):171-6. Sheng Li Ke Xue Jin Zhan. 2010. PMID: 21416975 Review. Chinese.
-
OPA1 requires mitofusin 1 to promote mitochondrial fusion.Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15927-32. doi: 10.1073/pnas.0407043101. Epub 2004 Oct 27. Proc Natl Acad Sci U S A. 2004. PMID: 15509649 Free PMC article.
-
Mitochondrial hyperfusion promotes NF-κB activation via the mitochondrial E3 ligase MULAN.FEBS J. 2014 Jul;281(14):3095-112. doi: 10.1111/febs.12846. Epub 2014 Jun 2. FEBS J. 2014. PMID: 24841215
-
Physiological functions of mitochondrial fusion.Ann N Y Acad Sci. 2010 Jul;1201:21-5. doi: 10.1111/j.1749-6632.2010.05615.x. Ann N Y Acad Sci. 2010. PMID: 20649534 Review.
Cited by
-
Mitochondrial quality control pathways as determinants of metabolic health.Bioessays. 2015 Aug;37(8):867-76. doi: 10.1002/bies.201500013. Epub 2015 May 26. Bioessays. 2015. PMID: 26010263 Free PMC article. Review.
-
Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission.J Cell Biol. 2015 Jan 5;208(1):109-23. doi: 10.1083/jcb.201404050. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547155 Free PMC article.
-
Morphological control of mitochondrial bioenergetics.Front Biosci (Landmark Ed). 2015 Jan 1;20(2):229-46. doi: 10.2741/4306. Front Biosci (Landmark Ed). 2015. PMID: 25553448 Free PMC article. Review.
-
Deceleration of fusion-fission cycles improves mitochondrial quality control during aging.PLoS Comput Biol. 2012;8(6):e1002576. doi: 10.1371/journal.pcbi.1002576. Epub 2012 Jun 28. PLoS Comput Biol. 2012. PMID: 22761564 Free PMC article.
-
Perspectives on: SGP symposium on mitochondrial physiology and medicine: what comes first, misshape or dysfunction? The view from metabolic excess.J Gen Physiol. 2012 Jun;139(6):455-63. doi: 10.1085/jgp.201210771. J Gen Physiol. 2012. PMID: 22641640 Free PMC article. No abstract available.
References
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 - PubMed
-
- Arnould T, Mercy L, Houbion A, Vankoningsloo S, Renard P, Pascal T, Ninane N, Demazy C, Raes M (2003) mtCLIC is up-regulated and maintains a mitochondrial membrane potential in mtDNA-depleted L929 cells. Faseb J 17: 2145–2147 - PubMed
-
- Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, Lenaers G (2007) OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res 313: 3800–3808 - PubMed
-
- Bowes T, Gupta RS (2008) Novel mitochondrial extensions provide evidence for a link between microtubule-directed movement and mitochondrial fission. Biochem Biophys Res Commun 376: 40–45 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

