The herpes simplex virus type 1 glycoprotein D (gD) cytoplasmic terminus and full-length gE are not essential and do not function in a redundant manner for cytoplasmic virion envelopment and egress
- PMID: 19357164
- PMCID: PMC2687392
- DOI: 10.1128/JVI.00128-09
The herpes simplex virus type 1 glycoprotein D (gD) cytoplasmic terminus and full-length gE are not essential and do not function in a redundant manner for cytoplasmic virion envelopment and egress
Abstract
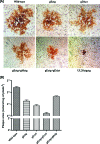
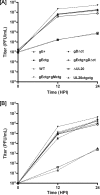
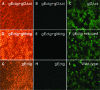
Herpes simplex virus type 1 (HSV-1) acquires its final envelope by budding into cytoplasmic vesicles thought to be derived from trans-Golgi network membranes. This process is facilitated by interactions among the carboxyl termini of viral glycoproteins and tegument proteins. To directly investigate the relative importance of the carboxyl terminus of glycoprotein D (gD) in the presence or absence of gE, a recombinant virus (gDDeltact) was constructed to specify a truncated gD lacking the carboxy-terminal 29 amino acids. Furthermore, two additional recombinant viruses were constructed by mutating from ATG to CTG the initiation codons of gE (gEctg) or both gE and gM (gEctg+gMctg), causing lack of expression of gE or both gE and gM, respectively. A fourth mutant virus was constructed to specify the gEctg+gDDeltact mutations. The replication properties of these viruses were compared to those of a newly constructed recombinant virus unable to express UL20 due to alteration of the two initiation codons of UL20 (UL20ctgctg). All recombinant viruses were constructed by using the double-Red, site-directed mutagenesis system implemented on the HSV-1(F) genome cloned into a bacterial artificial chromosome. The gEctg, gEctg+gMctg, gDDeltact, and gEctg+gDDeltact viruses produced viral plaques on African monkey kidney cells (Vero), as well as other cells, that were on average approximately 30 to 50% smaller than those produced by the wild-type virus HSV-1(F). In contrast, the UL20ctgctg virus produced very small plaques containing three to five cells, as reported previously for the DeltaUL20 virus lacking the entire UL20 gene. Viral replication kinetics of intracellular and extracellular viruses revealed that all recombinant viruses produced viral titers similar to those produced by the wild-type HSV-1(F) virus intracellularly and extracellularly at late times postinfection, with the exception of the UL20ctgctg and DeltaUL20 viruses, which replicated more than two-and-a-half logs less efficiently than HSV-1(F). Electron microscopy confirmed that all viruses, regardless of their different gene mutations, efficiently produced enveloped virions within infected cells, with the exception of the UL20ctgctg and DeltaUL20 viruses, which accumulated high levels of unenveloped virions in the cytoplasm. These results show that the carboxyl terminus of gD and the full-length gE, either alone or in a redundant manner, are not essential in cytoplasmic virion envelopment and egress from infected cells. Similarly, gM and gE do not function alone or in a redundant manner in cytoplasmic envelopment and virion egress, confirming previous findings.
Figures


Similar articles
-
Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment.J Virol. 2014 Jun;88(11):5927-35. doi: 10.1128/JVI.00278-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24600000 Free PMC article.
-
Functional hierarchy of herpes simplex virus 1 viral glycoproteins in cytoplasmic virion envelopment and egress.J Virol. 2012 Apr;86(8):4262-70. doi: 10.1128/JVI.06766-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318149 Free PMC article.
-
Phenylalanine residues at the carboxyl terminus of the herpes simplex virus 1 UL20 membrane protein regulate cytoplasmic virion envelopment and infectious virus production.J Virol. 2014 Jul;88(13):7618-27. doi: 10.1128/JVI.00657-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760889 Free PMC article.
-
Involvement of Terminase Complex in Herpes Simplex Virus Mature Virion Egress.Curr Protein Pept Sci. 2022;23(2):105-113. doi: 10.2174/1389203723666220217144432. Curr Protein Pept Sci. 2022. PMID: 35176987 Review.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
Cited by
-
Glycoprotein D of HSV-1 is dependent on tegument protein UL16 for packaging and contains a motif that is differentially required for syncytia formation.Virology. 2019 Jan 15;527:64-76. doi: 10.1016/j.virol.2018.09.018. Epub 2018 Nov 19. Virology. 2019. PMID: 30465930 Free PMC article.
-
Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment.J Virol. 2014 Jun;88(11):5927-35. doi: 10.1128/JVI.00278-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24600000 Free PMC article.
-
Functional hierarchy of herpes simplex virus 1 viral glycoproteins in cytoplasmic virion envelopment and egress.J Virol. 2012 Apr;86(8):4262-70. doi: 10.1128/JVI.06766-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318149 Free PMC article.
-
Phenylalanine residues at the carboxyl terminus of the herpes simplex virus 1 UL20 membrane protein regulate cytoplasmic virion envelopment and infectious virus production.J Virol. 2014 Jul;88(13):7618-27. doi: 10.1128/JVI.00657-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760889 Free PMC article.
-
Duck plague virus Glycoprotein J is functional but slightly impaired in viral replication and cell-to-cell spread.Sci Rep. 2018 Mar 6;8(1):4069. doi: 10.1038/s41598-018-22447-x. Sci Rep. 2018. PMID: 29511274 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

