Zebrafish genetic models for arrhythmia
- PMID: 19351520
- PMCID: PMC2836909
- DOI: 10.1016/j.pbiomolbio.2009.01.011
Zebrafish genetic models for arrhythmia
Abstract
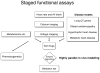
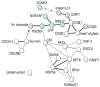
Over the last decade the zebrafish has emerged as a major genetic model organism. While stimulated originally by the utility of its transparent embryos for the study of vertebrate organogenesis, the success of the zebrafish was consolidated through multiple genetic screens, sequencing of the fish genome by the Sanger Center, and the advent of extensive genomic resources. In the last few years the potential of the zebrafish for in vivo cell biology, physiology, disease modeling and drug discovery has begun to be realized. This review will highlight work on cardiac electrophysiology, emphasizing the arenas in which the zebrafish complements other in vivo and in vitro models; developmental physiology, large-scale screens, high-throughput disease modeling and drug discovery. Much of this work is at an early stage, and so the focus will be on the general principles, the specific advantages of the zebrafish and on future potential.
Figures



Similar articles
-
Functional genomics in zebrafish as a tool to identify novel antiarrhythmic targets.Curr Med Chem. 2014;21(11):1320-9. doi: 10.2174/0929867321666131227130218. Curr Med Chem. 2014. PMID: 24372224 Review.
-
Chemical genetics: Drug screens in Zebrafish.Biosci Rep. 2005 Oct-Dec;25(5-6):289-97. doi: 10.1007/s10540-005-2891-8. Biosci Rep. 2005. PMID: 16307377 Review.
-
Model systems for the discovery and development of antiarrhythmic drugs.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):328-39. doi: 10.1016/j.pbiomolbio.2008.10.009. Epub 2008 Nov 11. Prog Biophys Mol Biol. 2008. PMID: 19038282 Review.
-
Inherited Ventricular Arrhythmia in Zebrafish: Genetic Models and Phenotyping Tools.Rev Physiol Biochem Pharmacol. 2023;184:33-68. doi: 10.1007/112_2021_65. Rev Physiol Biochem Pharmacol. 2023. PMID: 34533615 Review.
-
Deficient zebrafish ether-à-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants.Circulation. 2008 Feb 19;117(7):866-75. doi: 10.1161/CIRCULATIONAHA.107.752220. Epub 2008 Feb 4. Circulation. 2008. PMID: 18250272
Cited by
-
Optical mapping in the developing zebrafish heart.Pediatr Cardiol. 2012 Aug;33(6):916-22. doi: 10.1007/s00246-012-0300-1. Epub 2012 Mar 30. Pediatr Cardiol. 2012. PMID: 22460358 Review.
-
New and TALENted genome engineering toolbox.Circ Res. 2013 Aug 16;113(5):571-87. doi: 10.1161/CIRCRESAHA.113.301765. Circ Res. 2013. PMID: 23948583 Free PMC article. Review.
-
Studying synthetic lethal interactions in the zebrafish system: insight into disease genes and mechanisms.Dis Model Mech. 2012 Jan;5(1):33-7. doi: 10.1242/dmm.007989. Epub 2011 Nov 22. Dis Model Mech. 2012. PMID: 22107871 Free PMC article. Review.
-
Cardiovascular pharmacogenomics.Circ Res. 2011 Sep 16;109(7):807-20. doi: 10.1161/CIRCRESAHA.110.230995. Circ Res. 2011. PMID: 21921273 Free PMC article. Review.
-
An assay for lateral line regeneration in adult zebrafish.J Vis Exp. 2014 Apr 8;(86):51343. doi: 10.3791/51343. J Vis Exp. 2014. PMID: 24747778 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

