Two-Step Regulation of LAX PANICLE1 Protein Accumulation in Axillary Meristem Formation in Rice
- PMID: 19346465
- PMCID: PMC2685638
- DOI: 10.1105/tpc.108.065425
Two-Step Regulation of LAX PANICLE1 Protein Accumulation in Axillary Meristem Formation in Rice
Abstract
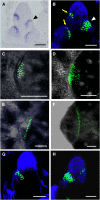
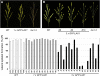
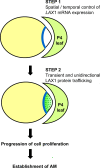
Axillary meristem (AM) formation is an important determinant of plant architecture. In rice (Oryza sativa), LAX PANICLE1 (LAX1) function is required for the generation of AM throughout the plant's lifespan. Here, we show a close relationship between AM initiation and leaf development; specifically, the plastochron 4 (P4) stage of leaf development is crucial for the proliferation of meristematic cells. Coincident with this, LAX1 expression starts in the axils of leaves at P4 stage. LAX1 mRNA accumulates in two to three layers of cells in the boundary region between the initiating AM and the shoot apical meristem. In lax1 mutants, the proliferation of meristematic cells is initiated but fails to progress into the formation of AM. The difference in sites of LAX1 mRNA expression and its action suggests non-cell-autonomous characteristics of LAX1 function. We found that LAX1 protein is trafficked to AM in a stage- and direction-specific manner. Furthermore, we present evidence that LAX1 protein movement is required for the full function of LAX1. Thus, we propose that LAX1 protein accumulates transiently in the initiating AM at P4 stage by a strict regulation of mRNA expression and a subsequent control of protein trafficking. This two-step regulation is crucial to the establishment of the new AM.
Figures



Comment in
-
Rice axillary meristem formation requires directional movement of LAX PANICLE1 protein.Plant Cell. 2009 Apr;21(4):1027. doi: 10.1105/tpc.109.210410. Epub 2009 Apr 3. Plant Cell. 2009. PMID: 19346463 Free PMC article. No abstract available.
Similar articles
-
LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems.Plant Cell. 2011 Sep;23(9):3276-87. doi: 10.1105/tpc.111.088765. Epub 2011 Sep 30. Plant Cell. 2011. PMID: 21963665 Free PMC article.
-
Phylogenomic analyses of the BARREN STALK1/LAX PANICLE1 (BA1/LAX1) genes and evidence for their roles during axillary meristem development.Mol Biol Evol. 2011 Jul;28(7):2147-59. doi: 10.1093/molbev/msr036. Epub 2011 Feb 5. Mol Biol Evol. 2011. PMID: 21297156
-
Rice axillary meristem formation requires directional movement of LAX PANICLE1 protein.Plant Cell. 2009 Apr;21(4):1027. doi: 10.1105/tpc.109.210410. Epub 2009 Apr 3. Plant Cell. 2009. PMID: 19346463 Free PMC article. No abstract available.
-
Regulation of axillary shoot development.Curr Opin Plant Biol. 2014 Feb;17:28-35. doi: 10.1016/j.pbi.2013.11.004. Epub 2013 Nov 27. Curr Opin Plant Biol. 2014. PMID: 24507491 Review.
-
Unraveling the molecular mechanisms governing axillary meristem initiation in plants.Planta. 2024 Mar 27;259(5):101. doi: 10.1007/s00425-024-04370-w. Planta. 2024. PMID: 38536474 Review.
Cited by
-
Conservation and divergence: Regulatory networks underlying reproductive branching in rice and maize.J Adv Res. 2022 Nov;41:179-190. doi: 10.1016/j.jare.2022.01.012. Epub 2022 Jan 29. J Adv Res. 2022. PMID: 36328747 Free PMC article. Review.
-
Two interacting basic helix-loop-helix transcription factors control flowering time in rice.Plant Physiol. 2023 May 2;192(1):205-221. doi: 10.1093/plphys/kiad077. Plant Physiol. 2023. PMID: 36756926 Free PMC article.
-
Is strigolactone signaling a key player in regulating tiller formation in response to nitrogen?Front Plant Sci. 2022 Dec 15;13:1081740. doi: 10.3389/fpls.2022.1081740. eCollection 2022. Front Plant Sci. 2022. PMID: 36589130 Free PMC article. No abstract available.
-
Characterization of the Molecular Events Underlying the Establishment of Axillary Meristem Region in Pepper.Int J Mol Sci. 2023 Aug 12;24(16):12718. doi: 10.3390/ijms241612718. Int J Mol Sci. 2023. PMID: 37628899 Free PMC article.
-
Comparative transcriptome analyses reveal different mechanism of high- and low-tillering genotypes controlling tiller growth in orchardgrass (Dactylis glomerata L.).BMC Plant Biol. 2020 Aug 5;20(1):369. doi: 10.1186/s12870-020-02582-2. BMC Plant Biol. 2020. PMID: 32758131 Free PMC article.
References
-
- Crawford, K.M., and Zambryski, P.C. (2000). Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10 1032–1040. - PubMed
-
- Ding, B., Itaya, A., and Qi, Y. (2003). Symplastic protein and RNA traffic: Regulatory proteins and regulatory factors. Curr. Opin. Plant Biol. 6 596–602. - PubMed
-
- Furutani, I., Sukegawa, S., and Kyozuka, J. (2006). Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J. 46 503–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

