Microfluidics and photonics for Bio-System-on-a-Chip: a review of advancements in technology towards a microfluidic flow cytometry chip
- PMID: 19343660
- PMCID: PMC2746115
- DOI: 10.1002/jbio.200810018
Microfluidics and photonics for Bio-System-on-a-Chip: a review of advancements in technology towards a microfluidic flow cytometry chip
Abstract
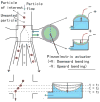
Microfluidics and photonics come together to form a field commonly referred to as 'optofluidics'. Flow cytometry provides the field with a technology base from which both microfluidic and photonic components be developed and integrated into a useful device. This article reviews some of the more recent developments to familiarize a reader with the current state of the technologies and also highlights the requirements of the device and how researchers are working to meet these needs.
Figures







Similar articles
-
Recent advances in miniaturized microfluidic flow cytometry for clinical use.Electrophoresis. 2007 Dec;28(24):4511-20. doi: 10.1002/elps.200700620. Electrophoresis. 2007. PMID: 18008312 Review.
-
Microfluidics: Sorting particles with light.Nat Mater. 2004 Jan;3(1):9-10. doi: 10.1038/nmat1041. Nat Mater. 2004. PMID: 14704776 No abstract available.
-
Micro-optics for microfluidic analytical applications.Chem Soc Rev. 2018 Feb 19;47(4):1391-1458. doi: 10.1039/c5cs00649j. Chem Soc Rev. 2018. PMID: 29308474 Review.
-
Developing optofluidic technology through the fusion of microfluidics and optics.Nature. 2006 Jul 27;442(7101):381-6. doi: 10.1038/nature05060. Nature. 2006. PMID: 16871205 Review.
-
On-chip flow cytometer using integrated photonics for the detection of human leukocytes.Sci Rep. 2024 May 20;14(1):10921. doi: 10.1038/s41598-024-60708-0. Sci Rep. 2024. PMID: 38769346 Free PMC article.
Cited by
-
Optofluidic device for label-free cell classification from whole blood.Lab Chip. 2012 Oct 7;12(19):3791-7. doi: 10.1039/c2lc40560a. Lab Chip. 2012. PMID: 22875178 Free PMC article.
-
Review Article: Recent advancements in optofluidic flow cytometer.Biomicrofluidics. 2010 Dec 30;4(4):43001. doi: 10.1063/1.3511706. Biomicrofluidics. 2010. PMID: 21267434 Free PMC article.
-
Review: imaging technologies for flow cytometry.Lab Chip. 2016 Nov 29;16(24):4639-4647. doi: 10.1039/c6lc01063f. Lab Chip. 2016. PMID: 27830849 Free PMC article. Review.
-
Applying an optical space-time coding method to enhance light scattering signals in microfluidic devices.Biomicrofluidics. 2011 Sep;5(3):34116-341166. doi: 10.1063/1.3624740. Epub 2011 Aug 16. Biomicrofluidics. 2011. PMID: 21915241 Free PMC article.
-
Optofluidic Tomography on a Chip.Appl Phys Lett. 2011 Apr 18;98(16):161109. doi: 10.1063/1.3548564. Epub 2011 Apr 20. Appl Phys Lett. 2011. PMID: 21580801 Free PMC article.
References
-
- Chen H, Meiners JC. Applied Physics Letters. 2004;84:2193.
-
- Beebe DJ, Mensing GA, Walker GM. Annual Review of Biomedical Engineering. 2002;4:261. - PubMed
-
- Groisman A, Enzelberger M, Quake SR. Microfluidic Memory and Control Devices. American Association for the Advancement of Science. 2003;300:955. - PubMed
-
- Urbanski JP, Thies W, Rhodes C, Amarasinghe S, Thorsen T. Lab on a Chip. 2006;6:96. - PubMed
-
- Prakash M, Gershenfeld N. Science. 2007;315:832. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

