Structural basis for the activation of muscle contraction by troponin and tropomyosin
- PMID: 19341744
- PMCID: PMC2693027
- DOI: 10.1016/j.jmb.2009.03.060
Structural basis for the activation of muscle contraction by troponin and tropomyosin
Abstract
The molecular regulation of striated muscle contraction couples the binding and dissociation of Ca(2+) on troponin (Tn) to the movement of tropomyosin on actin filaments. In turn, this process exposes or blocks myosin binding sites on actin, thereby controlling myosin crossbridge dynamics and consequently muscle contraction. Using 3D electron microscopy, we recently provided structural evidence that a C-terminal extension of TnI is anchored on actin at low Ca(2+) and competes with tropomyosin for a common site to drive tropomyosin to the B-state location, a constrained, relaxing position on actin that inhibits myosin-crossbridge association. Here, we show that release of this constraint at high Ca(2+) allows a second segment of troponin, probably representing parts of TnT or the troponin core domain, to promote tropomyosin movement on actin to the Ca(2+)-induced C-state location. With tropomyosin stabilized in this position, myosin binding interactions can begin. Tropomyosin appears to oscillate to a higher degree between respective B- and C-state positions on troponin-free filaments than on fully regulated filaments, suggesting that tropomyosin positioning in both states is troponin-dependent. By biasing tropomyosin to either of these two positions, troponin appears to have two distinct structural functions; in relaxed muscles at low Ca(2+), troponin operates as an inhibitor, while in activated muscles at high Ca(2+), it acts as a promoter to initiate contraction.
Figures

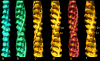
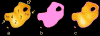

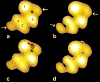
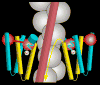
Similar articles
-
Structural basis for the regulation of muscle contraction by troponin and tropomyosin.J Mol Biol. 2008 Jun 20;379(5):929-35. doi: 10.1016/j.jmb.2008.04.062. Epub 2008 May 3. J Mol Biol. 2008. PMID: 18514658 Free PMC article.
-
The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments.Circ Res. 2010 Mar 5;106(4):705-11. doi: 10.1161/CIRCRESAHA.109.210047. Epub 2009 Dec 24. Circ Res. 2010. PMID: 20035081 Free PMC article.
-
A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle.J Struct Biol. 2006 Aug;155(2):273-84. doi: 10.1016/j.jsb.2006.02.020. Epub 2006 May 7. J Struct Biol. 2006. PMID: 16793285
-
Troponin Revealed: Uncovering the Structure of the Thin Filament On-Off Switch in Striated Muscle.Biophys J. 2021 Jan 5;120(1):1-9. doi: 10.1016/j.bpj.2020.11.014. Epub 2020 Nov 20. Biophys J. 2021. PMID: 33221250 Free PMC article. Review.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
Cited by
-
Structural dynamics of C-domain of cardiac troponin I protein in reconstituted thin filament.J Biol Chem. 2012 Mar 2;287(10):7661-74. doi: 10.1074/jbc.M111.281600. Epub 2011 Dec 28. J Biol Chem. 2012. PMID: 22207765 Free PMC article.
-
Cooperativity of myosin II motors in the non-regulated and regulated thin filaments investigated with high-speed AFM.J Gen Physiol. 2023 Mar 6;155(3):e202213190. doi: 10.1085/jgp.202213190. Epub 2023 Jan 12. J Gen Physiol. 2023. PMID: 36633585 Free PMC article.
-
The actin 'A-triad's' role in contractile regulation in health and disease.J Physiol. 2020 Jul;598(14):2897-2908. doi: 10.1113/JP276741. Epub 2019 Mar 28. J Physiol. 2020. PMID: 30770548 Free PMC article. Review.
-
Protein phosphorylation and signal transduction in cardiac thin filaments.J Biol Chem. 2011 Mar 25;286(12):9935-40. doi: 10.1074/jbc.R110.197731. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257760 Free PMC article. Review. No abstract available.
-
The N-terminal domains of myosin binding protein C can bind polymorphically to F-actin.J Mol Biol. 2011 Sep 23;412(3):379-86. doi: 10.1016/j.jmb.2011.07.056. Epub 2011 Jul 29. J Mol Biol. 2011. PMID: 21821050 Free PMC article.
References
-
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. - PubMed
-
- Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. - PubMed
-
- Moore PB, Huxley HE, DeRosier DJ. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970;50:279–292. - PubMed
-
- O’Brien EJ, Bennett PM, Hanson J. Optical diffraction studies of myofibrillar structure. Philos Trans Roy Soc Lond B Biol Sci. 1971;261:201–208. - PubMed
-
- Spudich JA, Huxley HE, Finch JT. The regulation of skeletal muscle contraction. II Structural studies of the interaction of the tropomyosin-troponin complex with actin. J Mol Biol. 1972;72:619–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL63774/HL/NHLBI NIH HHS/United States
- R01 HL063774/HL/NHLBI NIH HHS/United States
- HL38834/HL/NHLBI NIH HHS/United States
- AR34711/AR/NIAMS NIH HHS/United States
- RR08426/RR/NCRR NIH HHS/United States
- R37 HL036153/HL/NHLBI NIH HHS/United States
- HL36153/HL/NHLBI NIH HHS/United States
- P01 HL086655-020003/HL/NHLBI NIH HHS/United States
- R37 HL036153-19/HL/NHLBI NIH HHS/United States
- HL86655/HL/NHLBI NIH HHS/United States
- R01 HL036153/HL/NHLBI NIH HHS/United States
- R01 AR034711/AR/NIAMS NIH HHS/United States
- P01 HL086655/HL/NHLBI NIH HHS/United States
- R01 HL038834/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

