Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis
- PMID: 19339486
- PMCID: PMC2691048
- DOI: 10.1128/CVI.00005-09
Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis
Abstract
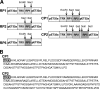
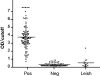
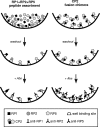
The aim of this work was to determine the best strategy to display antigens (Ags) on immunochemical devices to improve test selectivity and sensitivity. We comparatively evaluated five Trypanosoma cruzi antigenic recombinant peptides, chose the three more sensitive ones, built up chimeras bearing these selected Ags, and systematically compared by enzyme-linked immunosorbent assay the performance of the assortments of those peptides with that of the multiepitope constructions bearing all those peptides lineally fused. The better-performing Ags that were compared included peptides homologous to the previously described T. cruzi flagellar repetitive Ag (here named RP1), shed acute-phase Ag (RP2), B13 (RP5), and the chimeric recombinant proteins CP1 and CP2, bearing repetitions of RP1-RP2 and RP1-RP2-RP5, respectively. The diagnostic performances of these Ags were assessed for discrimination efficiency by the formula +OD/cutoff value (where +OD is the mean optical density value of the positive serum samples tested), in comparison with each other either alone, in mixtures, or as peptide-fused chimeras and with total parasite homogenate (TPH). The discrimination efficiency values obtained for CP1 and CP2 were 25% and 52% higher, respectively, than those of their individual-Ag mixtures. CP2 was the only Ag that showed enhanced discrimination efficiency between Chagas' disease-positive and -negative samples, compared with TPH. This study highlights the convenience of performing immunochemical assays using hybrid, single-molecule, chimeric Ags instead of peptide mixtures. CP2 preliminary tests rendered 98.6% sensitivity when evaluated with a 141-Chagas' disease-positive serum sample panel and 99.4% specificity when assessed with a 164-Chagas' disease-negative serum sample panel containing 15 samples from individuals infected with Leishmania spp.
Figures
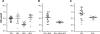
Similar articles
-
Recombinant multiepitope proteins expressed in Escherichia coli cells and their potential for immunodiagnosis.Microb Cell Fact. 2024 May 22;23(1):145. doi: 10.1186/s12934-024-02418-w. Microb Cell Fact. 2024. PMID: 38778337 Free PMC article. Review.
-
Immunoagglutination test to diagnose Chagas disease: comparison of different latex-antigen complexes.Trop Med Int Health. 2014 Nov;19(11):1346-54. doi: 10.1111/tmi.12379. Epub 2014 Aug 30. Trop Med Int Health. 2014. PMID: 25175083
-
Evaluation of a recombinant Trypanosoma cruzi mucin-like antigen for serodiagnosis of Chagas' disease.Clin Vaccine Immunol. 2011 Nov;18(11):1850-5. doi: 10.1128/CVI.05289-11. Epub 2011 Aug 31. Clin Vaccine Immunol. 2011. PMID: 21880857 Free PMC article.
-
Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp.Clin Vaccine Immunol. 2007 Aug;14(8):1045-9. doi: 10.1128/CVI.00127-07. Epub 2007 May 23. Clin Vaccine Immunol. 2007. PMID: 17522327 Free PMC article.
-
A Review on the use of Synthetic and Recombinant Antigens for the Immunodiagnosis of Tegumentary Leishmaniasis.Curr Med Chem. 2024;31(30):4763-4780. doi: 10.2174/0109298673298705240311114203. Curr Med Chem. 2024. PMID: 38509682 Free PMC article. Review.
Cited by
-
Development of a New Lateral Flow Assay Based on IBMP-8.1 and IBMP-8.4 Chimeric Antigens to Diagnose Chagas Disease.Biomed Res Int. 2020 Aug 17;2020:1803515. doi: 10.1155/2020/1803515. eCollection 2020. Biomed Res Int. 2020. PMID: 32908871 Free PMC article.
-
The use of peptides for immunodiagnosis of human Chagas disease.Amino Acids. 2024 May 2;56(1):35. doi: 10.1007/s00726-024-03394-6. Amino Acids. 2024. PMID: 38698213 Free PMC article. Review.
-
Chronic Chagas Disease Diagnosis: A Comparative Performance of Commercial Enzyme Immunoassay Tests.Am J Trop Med Hyg. 2016 May 4;94(5):1034-9. doi: 10.4269/ajtmh.15-0820. Epub 2016 Mar 14. Am J Trop Med Hyg. 2016. PMID: 26976886 Free PMC article.
-
Assessment of Liaison XL Murex Chagas diagnostic performance in blood screening for Chagas disease using a reference array of chimeric antigens.Transfusion. 2021 Sep;61(9):2701-2709. doi: 10.1111/trf.16583. Epub 2021 Jul 9. Transfusion. 2021. PMID: 34240750 Free PMC article.
-
Recombinant multiepitope proteins expressed in Escherichia coli cells and their potential for immunodiagnosis.Microb Cell Fact. 2024 May 22;23(1):145. doi: 10.1186/s12934-024-02418-w. Microb Cell Fact. 2024. PMID: 38778337 Free PMC article. Review.
References
-
- Aguillon, J. C., R. Harris, M. C. Molina, A. Colombo, C. Cortes, T. Hermosilla, P. Carreno, A. Orn, and A. Ferreira. 1997. Recognition of an immunogenetically selected Trypanosoma cruzi antigen by seropositive chagasic human sera. Acta Trop. 63159-166. - PubMed
-
- Almeida, E., M. A. Krieger, M. R. Carvalho, W. Oelemann, and S. Goldenberg. 1990. Use of recombinant antigens for the diagnosis of Chagas disease and blood bank screening. Mem. Inst. Oswaldo Cruz 85513-517. - PubMed
-
- Anandarao, R., S. Swaminathan, S. Fernando, A. M. Jana, and N. Khanna. 2005. A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr. Purif. 41136-147. - PubMed
-
- Aubert, D., G. T. Maine, J. C. Hunt, L. Howard, M. Sheu, S. Brojanac, L. E. Chovan, S. F. Nowlan, and J. M. Pinon. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 381144-1150. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

