San-Huang-Xie-Xin-Tang Protects against Activated Microglia- and 6-OHDA-Induced Toxicity in Neuronal SH-SY5Y Cells
- PMID: 19339484
- PMCID: PMC3135633
- DOI: 10.1093/ecam/nep025
San-Huang-Xie-Xin-Tang Protects against Activated Microglia- and 6-OHDA-Induced Toxicity in Neuronal SH-SY5Y Cells
Abstract
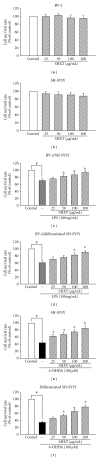
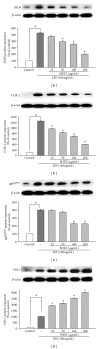
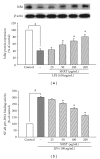
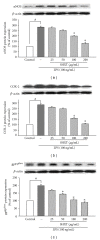
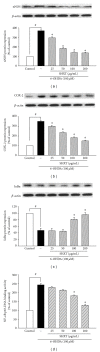
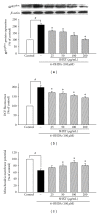
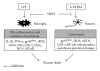
San-Huang-Xie-Xin-Tang (SHXT), composed of Coptidis rhizoma, Scutellariae radix and Rhei rhizoma, is a traditional Chinese herbal medicine used to treat gastritis, gastric bleeding and peptic ulcers. This study investigated the neuroprotective effects of SHXT on microglia-mediated neurotoxicity using co-cultured lipopolysaccharide (LPS)-activated microglia-like BV-2 cells with neuroblastoma SH-SY5Y cells. Effects of SHXT on 6-hydroxydopamine (6-OHDA)-induced neurotoxicity were also examined in SH-SY5Y cells. Results indicated SHXT inhibited LPS-induced inflammation of BV-2 cells by downregulation of iNOS, NO, COX-2, PGE(2), gp91(phox), iROS, TNF-α, IL-1β, inhibition of IκBα degradation and upregulation of HO-1. In addition, SHXT increased cell viability and down regulated nNOS, COX-2 and gp91(phox) of SH-SY5Y cells co-cultured with LPS activated BV-2 cells. SHXT treatment increased cell viability and mitochondria membrane potential (MMP), decreased expression of nNOS, COX-2, gp91(phox) and iROS, and inhibited IκBα degradation in 6-OHDA-treated SH-SY5Y cells. SHXT also attenuated LPS activated BV-2 cells- and 6-OHDA-induced cell death in differentiated SH-SY5Y cells with db-cAMP. Furthermore, SHXT-inhibited nuclear translocation of p65 subunit of NF-κB in LPS treated BV-2 cells and 6-OHDA treated SH-SY5Y cells. In conclusion, SHXT showed protection from activated microglia- and 6-OHDA-induced neurotoxicity by attenuating inflammation and oxidative stress.
Figures
Similar articles
-
Neuroprotective effects of glyceryl nonivamide against microglia-like cells and 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells.J Pharmacol Exp Ther. 2007 Dec;323(3):877-87. doi: 10.1124/jpet.107.125955. Epub 2007 Sep 12. J Pharmacol Exp Ther. 2007. PMID: 17855475
-
Neuroprotective Effects of San-Huang-Xie-Xin-Tang in the MPP(+)/MPTP Models of Parkinson's Disease In Vitro and In Vivo.Evid Based Complement Alternat Med. 2012;2012:501032. doi: 10.1155/2012/501032. Epub 2012 Mar 5. Evid Based Complement Alternat Med. 2012. PMID: 22474505 Free PMC article.
-
San-Huang-Xie-Xin-Tang reduces lipopolysaccharides-induced hypotension and inflammatory mediators.J Ethnopharmacol. 2005 Jan 4;96(1-2):99-106. doi: 10.1016/j.jep.2004.09.023. J Ethnopharmacol. 2005. PMID: 15588656
-
In vitro effects of Helicobacter pylori-induced infection in gastric epithelial AGS cells on microglia-mediated toxicity in neuroblastoma SH-SY5Y cells.Inflamm Res. 2009 Jun;58(6):329-35. doi: 10.1007/s00011-009-8075-4. Inflamm Res. 2009. PMID: 19247579
-
Effects of San-Huang-Xie-Xin-Tang on U46619-induced increase in pulmonary arterial blood pressure.J Ethnopharmacol. 2008 May 22;117(3):457-62. doi: 10.1016/j.jep.2008.02.024. Epub 2008 Feb 23. J Ethnopharmacol. 2008. PMID: 18387761
Cited by
-
Neuroprotective Activity of Coptisine from Coptis chinensis (Franch).Evid Based Complement Alternat Med. 2015;2015:827308. doi: 10.1155/2015/827308. Epub 2015 Jul 2. Evid Based Complement Alternat Med. 2015. PMID: 26229546 Free PMC article.
-
Echinacoside Protects against 6-Hydroxydopamine-Induced Mitochondrial Dysfunction and Inflammatory Responses in PC12 Cells via Reducing ROS Production.Evid Based Complement Alternat Med. 2015;2015:189239. doi: 10.1155/2015/189239. Epub 2015 Feb 18. Evid Based Complement Alternat Med. 2015. PMID: 25788961 Free PMC article.
-
San-Huang-Xie-Xin-Tang Prevents Rat Hearts from Ischemia/Reperfusion-Induced Apoptosis through eNOS and MAPK Pathways.Evid Based Complement Alternat Med. 2011;2011:915051. doi: 10.1093/ecam/neq061. Epub 2011 Apr 14. Evid Based Complement Alternat Med. 2011. PMID: 21785641 Free PMC article.
-
Elucidation of the Mechanisms and Molecular Targets of Sanhuang Xiexin Decoction for Type 2 Diabetes Mellitus Based on Network Pharmacology.Biomed Res Int. 2020 Aug 10;2020:5848497. doi: 10.1155/2020/5848497. eCollection 2020. Biomed Res Int. 2020. PMID: 32851081 Free PMC article.
-
From omics to drug metabolism and high content screen of natural product in zebrafish: a new model for discovery of neuroactive compound.Evid Based Complement Alternat Med. 2012;2012:605303. doi: 10.1155/2012/605303. Epub 2012 Aug 5. Evid Based Complement Alternat Med. 2012. PMID: 22919414 Free PMC article.
References
-
- von Bernhardi R. Glial cell dysregulation: a new perspective on Alzheimer disease. Neurotoxicity Research. 2007;12(4):215–232. - PubMed
-
- Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: Lessons from multiple sclerosis. Current Medicinal Chemistry. 2007;14(27):2925–2936. - PubMed
-
- Kim SU, De Vellis J. Microglia in health and disease. Journal of Neuroscience Research. 2005;81(3):302–313. - PubMed
-
- Gao H-M, Jiang J, Wilson B, Zhang W, Hong J-S, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. Journal of Neurochemistry. 2002;81(6):1285–1297. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

