Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients
- PMID: 19336761
- PMCID: PMC2686180
- DOI: 10.1182/blood-2008-09-176412
Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients
Abstract
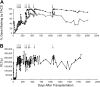
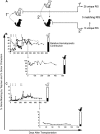
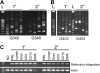
Overexpression of methylguanine methyltransferase P140K (MGMTP140K) has been successfully used for in vivo selection and chemoprotection in mouse and large animal studies, and has promise for autologous and allogeneic gene therapy. We examined the long-term safety of MGMTP140K selection in a clinically relevant dog model. Based on the association of provirus integration and proto-oncogene activation leading to leukemia in the X-linked immunodeficiency trial, we focused our analysis on the distribution of retrovirus integration sites (RIS) relative to proto-oncogene transcription start sites (TSS). We analyzed RIS near proto-oncogene TSS before (n = 157) and after (n = 129) chemotherapy in dogs that received MGMTP140K gene-modified cells and identified no overall increase of RIS near proto-oncogene TSS after chemotherapy. We also wanted to determine whether in vivo selected cells retained fundamental characteristics of hematopoietic stem cells. To that end, we performed secondary transplantation of MGMTP140K gene-modified cells after in vivo selection in dog leukocyte antigen (DLA)-matched dogs. Gene-modified cells achieved multilineage repopulation, and we identified the same gene-modified clone in both dogs more than 800 and 900 days after transplantation. These data suggest that MGMTP140K selection is well tolerated and should allow clinically for selection of gene-corrected cells in genetic or infectious diseases or chemoprotection for treatment of malignancy.
Figures


Similar articles
-
Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model.J Clin Invest. 2003 Nov;112(10):1581-8. doi: 10.1172/JCI18782. J Clin Invest. 2003. PMID: 14617759 Free PMC article.
-
In vivo selection of hematopoietic stem cells transduced at a low multiplicity-of-infection with a foamy viral MGMT(P140K) vector.Exp Hematol. 2008 Mar;36(3):283-92. doi: 10.1016/j.exphem.2007.11.009. Exp Hematol. 2008. PMID: 18279716 Free PMC article.
-
Characterisation of a P140K mutant O6-methylguanine-DNA-methyltransferase (MGMT)-expressing transgenic mouse line with drug-selectable bone marrow.J Gene Med. 2006 Sep;8(9):1071-85. doi: 10.1002/jgm.937. J Gene Med. 2006. PMID: 16927363
-
Large animal models for foamy virus vector gene therapy.Viruses. 2012 Dec 7;4(12):3572-88. doi: 10.3390/v4123572. Viruses. 2012. PMID: 23223198 Free PMC article. Review.
-
Vector design for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells.DNA Repair (Amst). 2007 Aug 1;6(8):1187-96. doi: 10.1016/j.dnarep.2007.03.017. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17482894 Free PMC article. Review.
Cited by
-
Foamy viral vector integration sites in SCID-repopulating cells after MGMTP140K-mediated in vivo selection.Gene Ther. 2015 Jul;22(7):591-5. doi: 10.1038/gt.2015.20. Epub 2015 Mar 19. Gene Ther. 2015. PMID: 25786870 Free PMC article.
-
Imaging stem cell-derived persistent foci after in vivo selection of lentiviral MGMT-P140K transduced murine bone marrow cells.Mol Ther. 2011 Jul;19(7):1342-52. doi: 10.1038/mt.2010.315. Epub 2011 Feb 8. Mol Ther. 2011. PMID: 21304493 Free PMC article.
-
Novel reporter systems for facile evaluation of I-SceI-mediated genome editing.Nucleic Acids Res. 2012 Jan;40(2):e14. doi: 10.1093/nar/gkr897. Epub 2011 Nov 21. Nucleic Acids Res. 2012. PMID: 22110042 Free PMC article.
-
Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates.J Clin Invest. 2010 Jul;120(7):2345-54. doi: 10.1172/JCI40767. Epub 2010 Jun 14. J Clin Invest. 2010. PMID: 20551514 Free PMC article.
-
A Nonhuman Primate Transplantation Model to Evaluate Hematopoietic Stem Cell Gene Editing Strategies for β-Hemoglobinopathies.Mol Ther Methods Clin Dev. 2017 Nov 21;8:75-86. doi: 10.1016/j.omtm.2017.11.005. eCollection 2018 Mar 16. Mol Ther Methods Clin Dev. 2017. PMID: 29276718 Free PMC article.
References
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. - PubMed
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
-
- Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. - PubMed
-
- Trobridge G, Beard BC, Kiem H-P. Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther. 2005;16:1355–1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

