Oxidant alterations in CD16 expression are cytoskeletal induced
- PMID: 19333136
- PMCID: PMC2783368
- DOI: 10.1097/SHK.0b013e3181a72530
Oxidant alterations in CD16 expression are cytoskeletal induced
Abstract
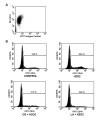
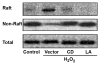
Oxidative stress during reperfusion of ischemia is associated with a phenotypic change in circulating monocytes from CD14++CD16- to a proinflammatory CD14+CD16+ subpopulation resulting in altered immunity and development of organ failure. However, the mechanism responsible remains unknown. We hypothesize that this phenotypic change, modeled by hydrogen peroxide exposure in vitro, is due to oxidative-induced intracellular calcium flux and distinct cytoskeletal and lipid raft changes. Peripheral blood monocytes obtained from healthy volunteers underwent 100 mM H2O2 exposure for 0 to 24 h. Selected cells were pretreated with 2 microM cytochalasin D, 1 microM lactrunculin A, or 30 microM 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid for 30 min. Cells underwent fluorescence-activated cell sorter for CD14, CD16, and cytokine expression. Cellular and lipid raft CD16 expression was determined by immunoblot and confocal microscopy. H2O2 exposed monocytes underwent a rapid time-dependent increase in the surface expression of CD16 from 12.81% +/- 3.53% to 37.12% +/- 7.61% at 24 h (P = 0.001). Total cellular CD16 was not changed by H2O2, but an increase in lipid raft and decrease in intracellular CD16 expression were seen after H2O2 exposure. This increase in CD16 expression was associated with a 27% increase in intracellular TNF-alpha, an alteration in actin polymerization, and the formation of raft macrodomains. These changes induced by H2O2 were inhibited by inhibition of actin polymerization (cytochalasin D and lactrunculin A) and intracellular calcium flux [1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid]. This study provides the first evidence that phenotypic alterations induced by oxidative stress during reperfusion may occur as a result of changes in cytoskeletal architecture due to calcium flux that result in lipid raft alterations rather than solely from demargination and/or production of bone marrow-derived CD16+ monocytes.
Figures





Similar articles
-
Cell-specific association of heat shock-induced proton flux with actin ring formation in Chenopodium cells: comparison of auto- and heterotroph cultures.Protoplasma. 2008 Dec;234(1-4):33-50. doi: 10.1007/s00709-008-0013-8. Epub 2008 Sep 20. Protoplasma. 2008. PMID: 18807117
-
The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF.J Immunol. 2002 Apr 1;168(7):3536-42. doi: 10.4049/jimmunol.168.7.3536. J Immunol. 2002. PMID: 11907116
-
Hydrogen peroxide-induced DNA damage is independent of nuclear calcium but dependent on redox-active ions.Biochem J. 1998 Oct 1;335 ( Pt 1)(Pt 1):85-94. doi: 10.1042/bj3350085. Biochem J. 1998. PMID: 9742216 Free PMC article.
-
Oxidative-induced calcium mobilization is dependent on annexin VI release from lipid rafts.Surgery. 2005 Aug;138(2):158-64. doi: 10.1016/j.surg.2005.03.018. Surgery. 2005. PMID: 16153422
-
Haemodialysis monocytopenia: differential sequestration kinetics of CD14+CD16+ and CD14++ blood monocyte subsets.Clin Exp Immunol. 2001 Jan;123(1):49-55. doi: 10.1046/j.1365-2249.2001.01436.x. Clin Exp Immunol. 2001. PMID: 11167997 Free PMC article.
Cited by
-
DNA methylation as an epigenetic mechanism in the regulation of LEDGF expression and biological response in aging and oxidative stress.Cell Death Discov. 2024 Jun 22;10(1):296. doi: 10.1038/s41420-024-02076-2. Cell Death Discov. 2024. PMID: 38909054 Free PMC article.
-
LPS-Induced Macrophage Activation and Plasma Membrane Fluidity Changes are Inhibited Under Oxidative Stress.J Membr Biol. 2016 Dec;249(6):789-800. doi: 10.1007/s00232-016-9927-9. Epub 2016 Sep 12. J Membr Biol. 2016. PMID: 27619206
-
A cytofluorometric study of membrane rafts in human monocyte subsets in atherosclerosis.Acta Naturae. 2014 Oct;6(4):80-8. Acta Naturae. 2014. PMID: 25558398 Free PMC article.
References
-
- Birkenmaier C, Hong YS, Horn JK. Modulation of the endotoxin receptor (CD14) in septic patients. J Trauma. 1992;32:473–478. discussion 478-479. - PubMed
-
- Laudanski K, De A, Brouxhon S, Kyrkanides S, Miller-Graziano C. Abnormal PGE(2) regulation of monocyte TNF-alpha levels in trauma patients parallels development of a more macrophage-like phenotype. Shock. 2004;22:204–212. - PubMed
-
- Rosseau S, Hammerl P, Maus U, Walmrath HD, Schutte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25–35. - PubMed
-
- Afessa B, Gajic O, Keegan MT. Severity of illness and organ failure assessment in adult intensive care units. Crit Care Clin. 2007;23:639–658. - PubMed
-
- Butt I, Shrestha BM. Two-hit hypothesis and multiple organ dysfunction syndrome. JNMA J Nepal Med Assoc. 2008;47:82–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

