TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells
- PMID: 19332490
- PMCID: PMC2714011
- DOI: 10.1113/jphysiol.2009.172254
TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells
Abstract
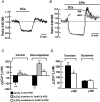
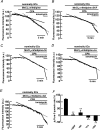
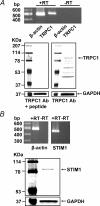
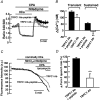
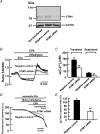
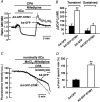
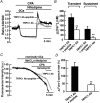
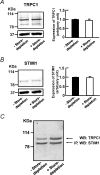
Previous studies in pulmonary arterial smooth muscle cells (PASMCs) showed that the TRPC1 channel mediates capacitative Ca(2+) entry (CCE), but the molecular signal(s) that activate TRPC1 in PASMCs remains unknown. The aim of the present study was to determine if TRPC1 mediates CCE through activation of STIM1 protein in mouse PASMCs. In primary cultured mouse PASMCs loaded with fura-2, cyclopiazonic acid (CPA) caused a transient followed by a sustained rise in intracellular Ca(2+) concentration ([Ca(2+)](i)). The transient but not the sustained rise in [Ca(2+)](i) was partially inhibited by nifedipine. In addition, CPA increased the rate of Mn(2+) quench of fura-2 fluorescence that was inhibited by SKF 96365, Ni(2+), La(3+) and Gd(3+), exhibiting pharmacological properties characteristic of CCE. The nifedipine-insensitive sustained rise in [Ca(2+)](i) and the increase in Mn(2+) quench of fura-2 fluorescence caused by CPA were both inhibited in cells pretreated with antibody raised against an extracellular epitope of TRPC1. Moreover, STIM1 siRNA reduced the rise in [Ca(2+)](i) and Mn(2+) quench of fura-2 fluorescence caused by CPA, whereas overexpression of STIM1 resulted in a marked increase in these responses. RT-PCR revealed TRPC1 and STIM1 mRNAs, and Western blot analysis identified TRPC1 and STIM1 proteins in mouse PASMCs. Furthermore, TRPC1 was found to co-immunoprecipitate with STIM1, and the precipitation level of TRPC1 was increased in cells subjected to store depletion. Taken together, store depletion causes activation of voltage-operated Ca(2+) entry and CCE. These data provide direct evidence that CCE is mediated by TRPC1 channel through activation of STIM1 in mouse PASMCs.
Figures

Similar articles
-
TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca(2+) entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells.Am J Physiol Cell Physiol. 2012 Dec 1;303(11):C1156-72. doi: 10.1152/ajpcell.00065.2012. Epub 2012 Oct 3. Am J Physiol Cell Physiol. 2012. PMID: 23034388
-
Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1079-90. doi: 10.1152/ajpcell.00548.2009. Epub 2010 Aug 25. Am J Physiol Cell Physiol. 2010. PMID: 20739625 Free PMC article.
-
Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells.Am J Physiol Cell Physiol. 2008 Jan;294(1):C313-23. doi: 10.1152/ajpcell.00258.2007. Epub 2007 Oct 31. Am J Physiol Cell Physiol. 2008. PMID: 17977940
-
The contribution of TRPC1 and STIM1 to capacitative Ca(2+) entry in pulmonary artery.Adv Exp Med Biol. 2010;661:123-35. doi: 10.1007/978-1-60761-500-2_8. Adv Exp Med Biol. 2010. PMID: 20204727 Review.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
Cited by
-
Hypoxic pulmonary vasoconstriction.Physiol Rev. 2012 Jan;92(1):367-520. doi: 10.1152/physrev.00041.2010. Physiol Rev. 2012. PMID: 22298659 Free PMC article. Review.
-
Hyperglycemia and Diabetes Downregulate the Functional Expression of TRPV4 Channels in Retinal Microvascular Endothelium.PLoS One. 2015 Jun 5;10(6):e0128359. doi: 10.1371/journal.pone.0128359. eCollection 2015. PLoS One. 2015. PMID: 26047504 Free PMC article.
-
Contribution and regulation of TRPC channels in store-operated Ca2+ entry.Curr Top Membr. 2013;71:149-79. doi: 10.1016/B978-0-12-407870-3.00007-X. Curr Top Membr. 2013. PMID: 23890115 Free PMC article. Review.
-
Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced relaxations and nitric oxide production in mesenteric arteries: comparative study using wild-type and TRPC1-/- mice.Channels (Austin). 2019 Dec;13(1):410-423. doi: 10.1080/19336950.2019.1673131. Epub 2019 Oct 11. Channels (Austin). 2019. PMID: 31603369 Free PMC article.
-
Protein kinases modulate store-operated channels in pulmonary artery smooth muscle cells.J Biomed Sci. 2011 Jan 6;18(1):2. doi: 10.1186/1423-0127-18-2. J Biomed Sci. 2011. PMID: 21211029 Free PMC article.
References
-
- Ahmmed GU, Mehta D, Stephen V, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Cα phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. - PubMed
-
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

