TSC-22 contributes to hematopoietic precursor cell proliferation and repopulation and is epigenetically silenced in large granular lymphocyte leukemia
- PMID: 19329776
- PMCID: PMC2689053
- DOI: 10.1182/blood-2009-02-205732
TSC-22 contributes to hematopoietic precursor cell proliferation and repopulation and is epigenetically silenced in large granular lymphocyte leukemia
Abstract
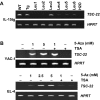
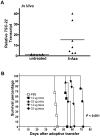
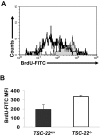
Aberrant methylation of tumor suppressor genes can lead to their silencing in many cancers. TSC-22 is a gene silenced in several solid tumors, but its function and the mechanism(s) responsible for its silencing are largely unknown. Here we demonstrate that the TSC-22 promoter is methylated in primary mouse T or natural killer (NK) large granular lymphocyte (LGL) leukemia and this is associated with down-regulation or silencing of TSC-22 expression. The TSC-22 deregulation was reversed in vivo by a 5-aza-2'-deoxycytidine therapy of T or NK LGL leukemia, which significantly increased survival of the mice bearing this disease. Ectopic expression of TSC-22 in mouse leukemia or lymphoma cell lines resulted in delayed in vivo tumor formation. Targeted disruption of TSC-22 in wild-type mice enhanced proliferation and in vivo repopulation efficiency of hematopoietic precursor cells (HPCs). Collectively, our data suggest that TSC-22 normally contributes to the regulation of HPC function and is a putative tumor suppressor gene that is hypermethylated and silenced in T or NK LGL leukemia.
Figures
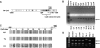



Similar articles
-
Dysregulated signaling, proliferation and apoptosis impact on the pathogenesis of TCRγδ+ T cell large granular lymphocyte leukemia.PLoS One. 2017 Apr 13;12(4):e0175670. doi: 10.1371/journal.pone.0175670. eCollection 2017. PLoS One. 2017. PMID: 28407008 Free PMC article.
-
Fibrosis and subsequent cytopenias are associated with basic fibroblast growth factor-deficient pluripotent mesenchymal stromal cells in large granular lymphocyte leukemia.J Immunol. 2013 Oct 1;191(7):3578-93. doi: 10.4049/jimmunol.1203424. Epub 2013 Sep 6. J Immunol. 2013. PMID: 24014875 Free PMC article.
-
Identification of a novel member of the snail/Gfi-1 repressor family, mlt 1, which is methylated and silenced in liver tumors of SV40 T antigen transgenic mice.Cancer Res. 2001 Feb 1;61(3):1144-53. Cancer Res. 2001. PMID: 11221845
-
Mutations in the signal transducer and activator of transcription family of genes in cancer.Cancer Sci. 2018 Apr;109(4):926-933. doi: 10.1111/cas.13525. Epub 2018 Mar 2. Cancer Sci. 2018. PMID: 29417693 Free PMC article. Review.
-
LGL leukemia: from pathogenesis to treatment.Blood. 2017 Mar 2;129(9):1082-1094. doi: 10.1182/blood-2016-08-692590. Epub 2017 Jan 23. Blood. 2017. PMID: 28115367 Review.
Cited by
-
Crucial role of TSC-22 in preventing the proteasomal degradation of p53 in cervical cancer.PLoS One. 2012;7(8):e42006. doi: 10.1371/journal.pone.0042006. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870275 Free PMC article.
-
X chromosome-wide analysis identifies DNA methylation sites influenced by cigarette smoking.Clin Epigenetics. 2016 Feb 24;8:20. doi: 10.1186/s13148-016-0189-2. eCollection 2016. Clin Epigenetics. 2016. PMID: 26913089 Free PMC article.
-
Large granular lymphocyte disorders: new etiopathogenetic clues as a rationale for innovative therapeutic approaches.Haematologica. 2009 Oct;94(10):1341-5. doi: 10.3324/haematol.2009.012161. Haematologica. 2009. PMID: 19794080 Free PMC article.
-
Madm (Mlf1 adapter molecule) cooperates with Bunched A to promote growth in Drosophila.J Biol. 2010;9(1):9. doi: 10.1186/jbiol216. Epub 2010 Feb 11. J Biol. 2010. PMID: 20149264 Free PMC article.
-
NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human.J Clin Invest. 2011 Apr;121(4):1456-70. doi: 10.1172/JCI43242. J Clin Invest. 2011. PMID: 21364281 Free PMC article.
References
-
- Shibanuma M, Kuroki T, Nose K. Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor beta 1 and other growth factors. J Biol Chem. 1992;267:10219–10224. - PubMed
-
- Hamil KG, Hall SH. Cloning of rat Sertoli cell follicle-stimulating hormone primary response complementary deoxyribonucleic acid: regulation of TSC-22 gene expression. Endocrinology. 1994;134:1205–1212. - PubMed
-
- Jay P, Ji JW, Marsollier C, Taviaux S, Berge-Lefranc JL, Berta P. Cloning of the human homologue of the TGF beta-stimulated clone 22 gene. Biochem Biophys Res Commun. 1996;222:821–826. - PubMed
-
- Treisman JE, Lai ZC, Rubin GM. Shortsighted acts in the decapentaplegic pathway in Drosophila eye development and has homology to a mouse TGF-beta-responsive gene. Development. 1995;121:2835–2845. - PubMed
-
- Vogel P, Magert HJ, Cieslak A, Adermann K, Forssmann WG. hDIP—a potential transcriptional regulator related to murine TSC-22 and Drosophila shortsighted (shs)—is expressed in a large number of human tissues. Biochim Biophys Acta. 1996;1309:200–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

