Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin
- PMID: 19326073
- PMCID: PMC2837916
- DOI: 10.1007/s10059-009-0050-y
Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin
Abstract
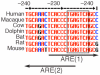
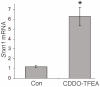
"Two-cysteine" peroxiredoxins are antioxidant enzymes that exert a cytoprotective effect in many models of oxidative stress. However, under highly oxidizing conditions they can be inactivated through hyperoxidation of their peroxidatic active site cysteine residue. Sulfiredoxin can reverse this hyperoxidation, thus reactivating peroxiredoxins. Here we review recent investigations that have shed further light on sulfiredoxin's role and regulation. Studies have revealed sulfiredoxin to be a dynamically regulated gene whose transcription is induced by a variety of signals and stimuli. Sulfiredoxin expression is regulated by the transcription factor AP-1, which mediates its up-regulation by synaptic activity in neurons, resulting in protection against oxidative stress. Furthermore, sulfiredoxin has been identified as a new member of the family of genes regulated by nuclear factor erythroid 2-related factor (Nrf2) via a conserved Aáë-acting antioxidant response element (ARE). As such, sulfiredoxin is likely to contribute to the net antioxidative effect of small molecule activators of Nrf2. As discussed here, the proximal AP-1 site of the sulfiredoxin promoter is embedded within the ARE, as is common with Nrf2 target genes. Other recent studies have shown that sulfiredoxin induction via Nrf2 may form an important part of the protective response to oxidative stress in the lung, preventing peroxiredoxin hyperoxidation and, in certain cases, subsequent degradation. We illustrate here that sulfiredoxin can be rapidly induced in vivo by administration of CDDO-TFEA, a synthetic triterpenoid inducer of endogenous Nrf2, which may offer a way of reversing peroxiredoxin hyperoxidation in vivo following chronic or acute oxidative stress.
Figures
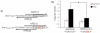
Similar articles
-
Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione.J Neurochem. 2008 Oct;107(2):533-43. doi: 10.1111/j.1471-4159.2008.05648.x. Epub 2008 Sep 16. J Neurochem. 2008. PMID: 18761713 Free PMC article.
-
Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1 and Nrf2.J Biol Chem. 2010 Nov 5;285(45):34419-28. doi: 10.1074/jbc.M110.126839. Epub 2010 Sep 7. J Biol Chem. 2010. PMID: 20826812 Free PMC article.
-
Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression.Free Radic Biol Med. 2012 Aug 1;53(3):447-56. doi: 10.1016/j.freeradbiomed.2012.05.020. Epub 2012 May 24. Free Radic Biol Med. 2012. PMID: 22634055 Review.
-
Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia.Antioxid Redox Signal. 2009 May;11(5):937-48. doi: 10.1089/ars.2008.2325. Antioxid Redox Signal. 2009. PMID: 19086807
-
Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin.Antioxid Redox Signal. 2011 Jul 1;15(1):99-109. doi: 10.1089/ars.2010.3564. Epub 2010 Dec 17. Antioxid Redox Signal. 2011. PMID: 20712415 Free PMC article. Review.
Cited by
-
Transcriptomic and innate immune responses to Yersinia pestis in the lymph node during bubonic plague.Infect Immun. 2010 Dec;78(12):5086-98. doi: 10.1128/IAI.00256-10. Epub 2010 Sep 27. Infect Immun. 2010. PMID: 20876291 Free PMC article.
-
SRXN1 Is Necessary for Resolution of GnRH-Induced Oxidative Stress and Induction of Gonadotropin Gene Expression.Endocrinology. 2019 Nov 1;160(11):2543-2555. doi: 10.1210/en.2019-00283. Endocrinology. 2019. PMID: 31504396 Free PMC article.
-
Reversal of high-glucose-induced transcriptional and epigenetic memories through NRF2 pathway activation.Life Sci Alliance. 2024 May 16;7(8):e202302382. doi: 10.26508/lsa.202302382. Print 2024 Aug. Life Sci Alliance. 2024. PMID: 38755006 Free PMC article.
-
Thioredoxin 1 protects astrocytes from oxidative stress by maintaining peroxiredoxin activity.Mol Med Rep. 2016 Mar;13(3):2864-70. doi: 10.3892/mmr.2016.4855. Epub 2016 Feb 3. Mol Med Rep. 2016. PMID: 26846911 Free PMC article.
-
Retinal oxidative stress activates the NRF2/ARE pathway: An early endogenous protective response to ocular hypertension.Redox Biol. 2021 Jun;42:101883. doi: 10.1016/j.redox.2021.101883. Epub 2021 Jan 29. Redox Biol. 2021. PMID: 33579667 Free PMC article.
References
-
- Bae SH, Woo HA, Sung SH, Lee HE, Lee SK, Kil IS, Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal. 2009 [Epub ahead of print]:PMID: 19086807. - PubMed
-
- Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. - PubMed
-
- Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–3097. - PubMed
-
- Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. - PubMed
-
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

