Phosphorylation modification of wheat lectin VER2 is associated with vernalization-induced O-GlcNAc signaling and intracellular motility
- PMID: 19287503
- PMCID: PMC2654674
- DOI: 10.1371/journal.pone.0004854
Phosphorylation modification of wheat lectin VER2 is associated with vernalization-induced O-GlcNAc signaling and intracellular motility
Abstract
Background: O-linked beta-N-acetylglucosamine (O-GlcNAc) modification of proteins mediates stress response and cellular motility in animal cells. The plant lectin concanavalin A can increase nuclear O-GlcNAc levels and decrease cytoplasmic O-GlcNAc levels in T lymphocytes. However, the functions of O-GlcNAc signaling in plants, as well as the relation between plant lectins and O-GlcNAc in response to environmental stimuli are largely undefined.
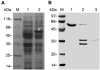
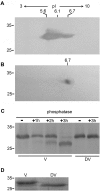
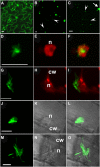
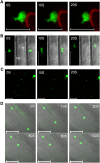
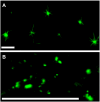
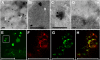
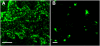
Methodology/principal findings: We describe a jacalin-like lectin VER2 in wheat that shows N-acetylglucosamine and galactose specificity. Immunocytochemical localization showed VER2 expression induced predominantly at potential nuclear structures in shoot tips and young leaves and weakly in cytoplasm in response to vernalization. In contrast, under devernalization (continuous stimulation with a higher temperature after vernalization), VER2 signals appeared predominantly in cytoplasm. 2-D electrophoresis, together with western blot analysis, showed phosphorylation modification of VER2 under vernalization. Immunoblot assay with O-GlcNAc-specific antibody revealed that vernalization increased O-GlcNAc modification of proteins at the global level. An O-GlcNAc-modified protein co-immunoprecipitated with VER2 in vernalized wheat plants but not in devernalized materials. The dynamic of VER2 was observed in transgenic Arabidopsis overexpressing the VER2-GFP fusion protein. Overexpressed VER2 accelerated nuclear migration. Immunogold labeling and indirect immunofluoresence colocalization assay indicated that VER2-GFP was targeted to the secretory pathway.
Conclusions/significance: O-GlcNAc signaling is involved in the vernalization response in wheat, and phosphorylation is necessary for the lectin VER2 involving O-GlcNAc signaling during vernalization. Our findings open the way to studies of O-GlcNAc protein modification in response to environmental signals in plants.
Conflict of interest statement
Figures


Similar articles
-
O-GlcNAc-mediated interaction between VER2 and TaGRP2 elicits TaVRN1 mRNA accumulation during vernalization in winter wheat.Nat Commun. 2014 Aug 5;5:4572. doi: 10.1038/ncomms5572. Nat Commun. 2014. PMID: 25091017 Free PMC article.
-
Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2.Planta. 2003 Jun;217(2):261-70. doi: 10.1007/s00425-003-0994-7. Epub 2003 Mar 4. Planta. 2003. PMID: 12783334
-
Nuclear localization of STAT5A modified with O-linked N-acetylglucosamine and early involution in the mammary gland of Hirosaki hairless rat.J Biol Chem. 2005 Dec 30;280(52):43010-6. doi: 10.1074/jbc.M509481200. Epub 2005 Oct 14. J Biol Chem. 2005. PMID: 16227201
-
Glycan-dependent signaling: O-linked N-acetylglucosamine.FASEB J. 2001 Sep;15(11):1865-76. doi: 10.1096/fj.01-0094rev. FASEB J. 2001. PMID: 11532966 Review.
-
The hexosamine signaling pathway: deciphering the "O-GlcNAc code".Sci STKE. 2005 Nov 29;2005(312):re13. doi: 10.1126/stke.3122005re13. Sci STKE. 2005. PMID: 16317114 Review.
Cited by
-
Dynamic eIF3a O-GlcNAcylation controls translation reinitiation during nutrient stress.Nat Chem Biol. 2022 Feb;18(2):134-141. doi: 10.1038/s41589-021-00913-4. Epub 2021 Dec 9. Nat Chem Biol. 2022. PMID: 34887587 Free PMC article.
-
Genome-wide identification and expression analysis of dirigent-jacalin genes from plant chimeric lectins in Moso bamboo (Phyllostachys edulis).PLoS One. 2021 Mar 16;16(3):e0248318. doi: 10.1371/journal.pone.0248318. eCollection 2021. PLoS One. 2021. PMID: 33724993 Free PMC article.
-
JACALIN-LECTIN LIKE1 Regulates the Nuclear Accumulation of GLYCINE-RICH RNA-BINDING PROTEIN7, Influencing the RNA Processing of FLOWERING LOCUS C Antisense Transcripts and Flowering Time in Arabidopsis.Plant Physiol. 2015 Nov;169(3):2102-17. doi: 10.1104/pp.15.00801. Epub 2015 Sep 21. Plant Physiol. 2015. PMID: 26392261 Free PMC article.
-
The Crystal Structure of the Defense Conferring Rice Protein OsJAC1 Reveals a Carbohydrate Binding Site on the Dirigent-like Domain.Biomolecules. 2022 Aug 17;12(8):1126. doi: 10.3390/biom12081126. Biomolecules. 2022. PMID: 36009020 Free PMC article.
-
Wheat genomic study for genetic improvement of traits in China.Sci China Life Sci. 2022 Sep;65(9):1718-1775. doi: 10.1007/s11427-022-2178-7. Epub 2022 Aug 24. Sci China Life Sci. 2022. PMID: 36018491 Review.
References
-
- Peumans WJ, Hause B, Van Damme EJ. The galactose-binding and mannose-binding jacalin-related lectins are located in different sub-cellular compartments. FEBS Lett. 2000;477:186–192. - PubMed
-
- Van Damme EJ, Lannoo N, Fouquaert E, Peumans WJ. The identification of inducible cytoplasmic/nuclear carbohydrate-binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj J. 2004;20:449–460. - PubMed
-
- Zhang W, Peumans WJ, Barre A, Astoul CH, Rovira P, et al. Isolation and characterization of a jacalin-related mannose-binding lectin from salt-stressed rice (Oryza sativa) plants. Planta. 2000;210:970–978. - PubMed
-
- Grunwald I, Heinig I, Thole HH, Neumann D, Kahmann U, et al. Purification and characterisation of a jacalin-related, coleoptile specific lectin from Hordeum vulgare. Planta. 2007;226:225–234. - PubMed
-
- Chen Y, Peumans WJ, Hause B, Bras J, Kumar M, et al. Jasmonic acid methyl ester induces the synthesis of a cytoplasmic/nuclear chito-oligosaccharide binding lectin in tobacco leaves. Faseb J. 2002;16:905–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

