Novel 2- and 4-substituted 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor
- PMID: 19284749
- PMCID: PMC2765805
- DOI: 10.1021/jm801659w
Novel 2- and 4-substituted 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor
Abstract
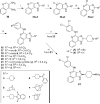
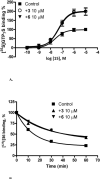
4-Arylamino and 2- cycloalkyl (including amino substitution) modifications were made in a series of 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the human A(3) adenosine receptor (AR). In addition to allosteric modulation of the maximum functional efficacy (in [(35)S]GTPgammaS G protein binding assay) of the A(3)AR agonist Cl-IB-MECA (15), some analogues also weakly inhibited equilibrium radioligand binding at ARs. 4-(3,5-Dichlorophenylamino) (6) or 2-(1-adamantyl) (20) substitution produced allosteric enhancement (twice the maximal agonist efficacy), with minimal inhibition of orthosteric AR binding. 2-(4-Tetrahydropyranyl) substitution abolished allosteric enhancement but preserved inhibition of orthosteric binding. Introduction of nitrogen in the six-membered ring at the 2 position, to improve aqueous solubility and provide a derivatization site, greatly reduced the allosteric enhancement. 2-(4-(Benzoylamino)cyclohexyl) analogues 23 and 24 were weak negative A(3)AR modulators. Thus, consistent with previous findings, the allosteric and orthosteric inhibitory A(3)AR effects in imidazoquinolines are structurally separable, suggesting the possible design of additional derivatives with enhanced positive or negative allosteric A(3)AR activity and improved selectivity in comparison to inhibition of orthosteric binding.
Figures
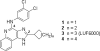
Similar articles
-
Structure-activity relationships of new 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric enhancers of the A3 adenosine receptor.J Med Chem. 2006 Jun 1;49(11):3354-61. doi: 10.1021/jm060086s. J Med Chem. 2006. PMID: 16722654 Free PMC article.
-
Flexible modulation of agonist efficacy at the human A3 adenosine receptor by the imidazoquinoline allosteric enhancer LUF6000.BMC Pharmacol. 2008 Dec 12;8:20. doi: 10.1186/1471-2210-8-20. BMC Pharmacol. 2008. PMID: 19077268 Free PMC article.
-
A series of 2,4-disubstituted quinolines as a new class of allosteric enhancers of the adenosine A3 receptor.J Med Chem. 2009 Feb 26;52(4):926-31. doi: 10.1021/jm8014052. J Med Chem. 2009. PMID: 19161279
-
Allosteric modulation of purine and pyrimidine receptors.Adv Pharmacol. 2011;61:187-220. doi: 10.1016/B978-0-12-385526-8.00007-2. Adv Pharmacol. 2011. PMID: 21586360 Free PMC article. Review.
-
Species dependence of A3 adenosine receptor pharmacology and function.Purinergic Signal. 2023 Sep;19(3):523-550. doi: 10.1007/s11302-022-09910-1. Epub 2022 Dec 20. Purinergic Signal. 2023. PMID: 36538251 Free PMC article. Review.
Cited by
-
Characterization of Dual-Acting A3 Adenosine Receptor Positive Allosteric Modulators That Preferentially Enhance Adenosine-Induced Gαi3 and GαoA Isoprotein Activation.ACS Pharmacol Transl Sci. 2022 Jul 15;5(8):625-641. doi: 10.1021/acsptsci.2c00076. eCollection 2022 Aug 12. ACS Pharmacol Transl Sci. 2022. PMID: 35983277 Free PMC article.
-
Functionally biased modulation of A(3) adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers.Biochem Pharmacol. 2011 Sep 15;82(6):658-68. doi: 10.1016/j.bcp.2011.06.017. Epub 2011 Jun 21. Biochem Pharmacol. 2011. PMID: 21718691 Free PMC article.
-
Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery.J Med Chem. 2012 Feb 23;55(4):1445-64. doi: 10.1021/jm201139r. Epub 2012 Jan 6. J Med Chem. 2012. PMID: 22148748 Free PMC article. No abstract available.
-
A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy.Med Res Rev. 2018 Jul;38(4):1031-1072. doi: 10.1002/med.21456. Epub 2017 Jul 6. Med Res Rev. 2018. PMID: 28682469 Free PMC article. Review.
-
Structure-Activity Studies of 1H-Imidazo[4,5-c]quinolin-4-amine Derivatives as A3 Adenosine Receptor Positive Allosteric Modulators.J Med Chem. 2022 Nov 24;65(22):15238-15262. doi: 10.1021/acs.jmedchem.2c01170. Epub 2022 Nov 11. J Med Chem. 2022. PMID: 36367749 Free PMC article.
References
-
- Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3'-aminoadenosine-5'-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J. Pharmacol. Exp. Ther. 2008;324:234–243. - PMC - PubMed
-
- Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm. Bowel Dis. 2006;12:766–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials

