Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa
- PMID: 19282780
- PMCID: PMC2674125
- DOI: 10.1097/QAI.0b013e318199072e
Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa
Abstract
Objective: To determine adherence to and effectiveness of antiretroviral therapy (ART) in adolescents vs. adults in southern Africa.
Design: Observational cohort study.
Setting: Aid for AIDS, a private sector disease management program in southern Africa.
Subjects: Adolescents (age 11-19 years; n = 154) and adults (n = 7622) initiating ART between 1999 and 2006 and having a viral load measurement within 1 year after ART initiation.
Main outcome measures: Primary: virologic suppression (HIV viral load < or = 400 copies/mL), viral rebound, and CD4 T-cell count at 6, 12, 18, and 24 months after ART initiation. Secondary: adherence assessed by pharmacy refills at 6, 12, and 24 months. Multivariate analyses: loglinear regression and Cox proportional hazards.
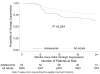
Results: A significantly smaller proportion of adolescents achieved 100% adherence at each time point (adolescents: 20.7% at 6 months, 14.3% at 12 months, and 6.6% at 24 months; adults: 40.5%, 27.9%, and 20.6% at each time point, respectively; P < 0.01). Patients achieving 100% 12-month adherence were significantly more likely to exhibit virologic suppression at 12 months, regardless of age. However, adolescents achieving virologic suppression had significantly shorter time to viral rebound (adjusted hazard ratio 2.03; 95% confidence interval: 1.31 to 3.13; P < 0.003). Adolescents were less likely to experience long-term immunologic recovery despite initial CD4 T-cell counts comparable to adults.
Conclusions: Compared with adults, adolescents in southern Africa are less adherent to ART and have lower rates of virologic suppression and immunologic recovery and a higher rate of virologic rebound after initial suppression. Studies must determine specific barriers to adherence in this population and develop appropriate interventions.
Conflict of interest statement
Conflict of interest
Figures
Similar articles
-
Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes.Ann Intern Med. 2007 Apr 17;146(8):564-73. doi: 10.7326/0003-4819-146-8-200704170-00007. Ann Intern Med. 2007. PMID: 17438315
-
Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults.AIDS. 2008 Oct 18;22(16):2117-25. doi: 10.1097/QAD.0b013e328310407e. AIDS. 2008. PMID: 18832875 Free PMC article.
-
Optimal monitoring strategies for guiding when to switch first-line antiretroviral therapy regimens for treatment failure in adults and adolescents living with HIV in low-resource settings.Cochrane Database Syst Rev. 2010 Apr 14;(4):CD008494. doi: 10.1002/14651858.CD008494. Cochrane Database Syst Rev. 2010. PMID: 20393969 Review.
-
Younger Age Predicts Failure to Achieve Viral Suppression and Virologic Rebound Among HIV-1-Infected Persons in Serodiscordant Partnerships.AIDS Res Hum Retroviruses. 2016 Feb;32(2):148-54. doi: 10.1089/AID.2015.0296. AIDS Res Hum Retroviruses. 2016. PMID: 26670218 Free PMC article.
-
A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults.AIDS Behav. 2013 Jan;17(1):41-60. doi: 10.1007/s10461-012-0159-4. AIDS Behav. 2013. PMID: 22411426 Review.
Cited by
-
HIV Care Cascade Among Adolescents in a "Test and Treat" Community-Based Intervention: HPTN 071 (PopART) for Youth Study.J Adolesc Health. 2021 Apr;68(4):719-727. doi: 10.1016/j.jadohealth.2020.07.029. Epub 2020 Oct 12. J Adolesc Health. 2021. PMID: 33059959 Free PMC article. Clinical Trial.
-
A non-specialist depression care pathway for adolescents living with HIV and transitioning into adult care in Peru: a nested, proof of concept pilot study.Glob Ment Health (Camb). 2021 May 26;8:e17. doi: 10.1017/gmh.2021.16. eCollection 2021. Glob Ment Health (Camb). 2021. PMID: 34104457 Free PMC article.
-
The missed HIV-positive children of Ethiopia.PLoS One. 2015 Apr 16;10(4):e0124041. doi: 10.1371/journal.pone.0124041. eCollection 2015. PLoS One. 2015. PMID: 25879446 Free PMC article.
-
Impact of Family-Based Economic Empowerment Intervention, Suubi+Adherence (2012-2018) on Multidimensional Poverty for Adolescents Living with HIV (ALWHIV) in Uganda.Int J Environ Res Public Health. 2022 Nov 2;19(21):14326. doi: 10.3390/ijerph192114326. Int J Environ Res Public Health. 2022. PMID: 36361203 Free PMC article. Clinical Trial.
-
Predictors of non adherence to antiretroviral therapy at an urban HIV care and treatment center in Tanzania.Drug Healthc Patient Saf. 2018 Aug 21;10:79-88. doi: 10.2147/DHPS.S143178. eCollection 2018. Drug Healthc Patient Saf. 2018. PMID: 30174460 Free PMC article.
References
-
- Markowitz M, Conant M, Hurley A, et al. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)–1 protease, to treat HIV infection. J Infect Dis. 1998;177:1533–1540. - PubMed
-
- Markowitz M, Saag M, Powderly WG, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. - PubMed
-
- Hirsch M, Steigbigel R, Staszewski S, et al. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced human immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis. 1999;180:659–665. - PubMed
-
- Staszewski S, Morales-Ramirez J, Tashima KT, et al. for the Study 006 Team. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med. 1999;341:1865–1873. - PubMed
-
- Starr SE, Fletcher CV, Spector SA, et al. for the Pediatric AIDS Clinical Trials Group 382 Team. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. N Engl J Med. 1999;341:1874–1881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials