Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells
- PMID: 19279100
- PMCID: PMC2682094
- DOI: 10.1128/JVI.02498-08
Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells
Abstract
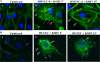
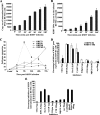
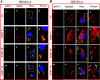
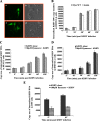
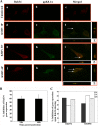
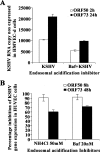
Kaposi's sarcoma-associated herpesvirus (KSHV) utilizes clathrin-mediated endocytosis for its infectious entry into human foreskin fibroblast (HFF) cells (S. M. Akula, P. P. Naranatt, N.-S. Walia, F.-Z. Wang, B. Fegley, and B. Chandran, J. Virol. 77:7978-7990, 2003). Here, we characterized KSHV entry into primary human microvascular dermal endothelial (HMVEC-d) and human umbilical vein endothelial (HUVEC) cells. Similar to the results for HMVEC-d cells, KSHV infection of HUVEC cells also resulted in an initial high level and subsequent decline in the expression of the lytic switch gene, ORF50, while latent gene expression persisted. Internalized virus particles enclosed in irregular vesicles were observed by electron microscopy of infected HMVEC-d cells. At an early time of infection, colocalization of KSHV capsid with envelope was observed by immunofluorescence analysis, thus demonstrating endocytosis of intact enveloped virus particles. Chlorpromazine, an inhibitor of clathrin-mediated endocytosis, and filipin (C(35)H(58)O(11)), a caveolar endocytosis inhibitor, did not have any effect on KSHV binding, entry (DNA internalization), or gene expression in HMVEC-d and HUVEC cells. In contrast to the results for HFF cells, virus entry and gene expression in both types of endothelial cells were significantly blocked by macropinocytosis inhibitors (EIPA [5-N-ethyl-N-isoproamiloride] and rottlerin [C(30)H(28)O(8)]) and by cytochalasin D, which affects actin polymerization. Inhibition of lipid raft blocked viral gene expression in HMVEC-d cells but not in HUVEC or HFF cells. In HMVEC-d and HUVEC cells, KSHV induced the actin polymerization and formation of lamellipodial extensions that are essential for macropinocytosis. Inhibition of macropinocytosis resulted in the distribution of viral capsids at the HMVEC-d cell periphery, and capsids did not associate with microtubules involved in the nuclear delivery of viral DNA. Internalized KSHV in HMVEC-d and HUVEC cells colocalized with the macropinocytosis marker dextran and not with the clathrin pathway marker transferrin or with caveolin. Dynasore, an inhibitor of dynamin, did not block viral entry into endothelial cells but did inhibit entry into HFF cells. KSHV was not associated with the early endosome marker EEA-1 in HMVEC-d cells, but rather with the late endosome marker LAMP1, as well as with Rab34 GTPase that is known to regulate macropinocytosis. Silencing Rab34 with small interfering RNA dramatically inhibited KSHV gene expression. Bafilomycin-mediated disruption of endosomal acidification inhibited viral gene expression. Taken together, these findings suggest that KSHV utilizes the actin polymerization-dependent, dynamin-independent macropinocytic pathway that involves a Rab34 GTPase-dependent late endosome and low-pH environment for its infectious entry into HMVEC-d and HUVEC cells. These studies also demonstrate that KSHV utilizes different modes of endocytic entry in fibroblast and endothelial cells.
Figures





Similar articles
-
Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells.PLoS Pathog. 2009 Jul;5(7):e1000512. doi: 10.1371/journal.ppat.1000512. Epub 2009 Jul 10. PLoS Pathog. 2009. PMID: 19593382 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection.J Virol. 2008 Dec;82(24):12126-44. doi: 10.1128/JVI.01146-08. Epub 2008 Oct 1. J Virol. 2008. PMID: 18829766 Free PMC article.
-
Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression.J Virol. 2004 Apr;78(7):3601-20. doi: 10.1128/jvi.78.7.3601-3620.2004. J Virol. 2004. PMID: 15016882 Free PMC article.
-
KSHV Entry and Trafficking in Target Cells-Hijacking of Cell Signal Pathways, Actin and Membrane Dynamics.Viruses. 2016 Nov 14;8(11):305. doi: 10.3390/v8110305. Viruses. 2016. PMID: 27854239 Free PMC article. Review.
-
Interaction of KSHV with host cell surface receptors and cell entry.Viruses. 2014 Oct 23;6(10):4024-46. doi: 10.3390/v6104024. Viruses. 2014. PMID: 25341665 Free PMC article. Review.
Cited by
-
Interaction of human tumor viruses with host cell surface receptors and cell entry.Viruses. 2015 May 22;7(5):2592-617. doi: 10.3390/v7052592. Viruses. 2015. PMID: 26008702 Free PMC article. Review.
-
Endocytic Pathways Used by Andes Virus to Enter Primary Human Lung Endothelial Cells.PLoS One. 2016 Oct 25;11(10):e0164768. doi: 10.1371/journal.pone.0164768. eCollection 2016. PLoS One. 2016. PMID: 27780263 Free PMC article.
-
Human Ebola virus infection in West Africa: a review of available therapeutic agents that target different steps of the life cycle of Ebola virus.Infect Dis Poverty. 2014 Nov 28;3:43. doi: 10.1186/2049-9957-3-43. eCollection 2014. Infect Dis Poverty. 2014. PMID: 25699183 Free PMC article.
-
Infectious bronchitis virus entry mainly depends on clathrin mediated endocytosis and requires classical endosomal/lysosomal system.Virology. 2019 Feb;528:118-136. doi: 10.1016/j.virol.2018.12.012. Epub 2018 Dec 28. Virology. 2019. PMID: 30597347 Free PMC article.
-
KSHV: pathways to tumorigenesis and persistent infection.Adv Virus Res. 2014;88:111-59. doi: 10.1016/B978-0-12-800098-4.00002-7. Adv Virus Res. 2014. PMID: 24373311 Free PMC article. Review.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

