Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation
- PMID: 19278624
- PMCID: PMC2697849
- DOI: 10.1016/j.biochi.2009.01.010
Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation
Abstract
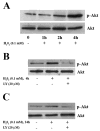
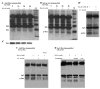
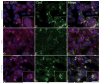
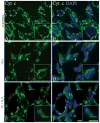
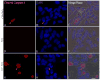
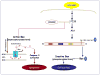
Reactive oxygen species such as hydrogen peroxide (H(2)O(2)) are involved in many cellular processes that positively and negatively regulate cell fate. H(2)O(2), acting as an intracellular messenger, activates phosphatidylinositol-3 kinase (PI3K) and its downstream target Akt, and promotes cell survival. The aim of the current study was to understand the mechanism by which PI3K/Akt signaling promotes survival in SH-SY5Y neuroblastoma cells. We demonstrate that PI3K/Akt mediates phosphorylation of the pro-apoptotic Bcl-2 family member Bax. This phosphorylation suppresses apoptosis and promotes cell survival. Increased survival in the presence of H(2)O(2) was blocked by LY294002, an inhibitor of PI3K activation. LY294002 prevented Bax phosphorylation and resulted in Bax translocation to the mitochondria, cytochrome c release, caspase-3 activation, and cell death. Collectively, these findings reveal a mechanism by which H(2)O(2)-induced activation of PI3K/Akt influences post-translational modification of Bax and inactivates a key component of the cell death machinery.
Figures
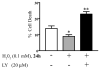
Similar articles
-
Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax.Eur J Pharmacol. 2010 Feb 25;628(1-3):155-63. doi: 10.1016/j.ejphar.2009.11.046. Epub 2009 Dec 3. Eur J Pharmacol. 2010. PMID: 19962976
-
The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria.J Biol Chem. 2002 Apr 19;277(16):14040-7. doi: 10.1074/jbc.M108975200. Epub 2002 Feb 12. J Biol Chem. 2002. PMID: 11842081
-
Nicotine inactivation of the proapoptotic function of Bax through phosphorylation.J Biol Chem. 2005 Mar 18;280(11):10781-9. doi: 10.1074/jbc.M500084200. Epub 2005 Jan 10. J Biol Chem. 2005. PMID: 15642728
-
25-Hydroxyl-protopanaxatriol protects against H2O2-induced H9c2 cardiomyocytes injury via PI3K/Akt pathway and apoptotic protein down-regulation.Biomed Pharmacother. 2018 Mar;99:33-42. doi: 10.1016/j.biopha.2018.01.039. Epub 2018 Jan 8. Biomed Pharmacother. 2018. PMID: 29324310
-
17β-estradiol impedes Bax-involved mitochondrial apoptosis of retinal nerve cells induced by oxidative damage via the phosphatidylinositol 3-kinase/Akt signal pathway.J Mol Neurosci. 2013 Jul;50(3):482-93. doi: 10.1007/s12031-013-9968-9. Epub 2013 Jan 30. J Mol Neurosci. 2013. PMID: 23361188
Cited by
-
ANXA11 regulates the tumorigenesis, lymph node metastasis and 5-fluorouracil sensitivity of murine hepatocarcinoma Hca-P cells by targeting c-Jun.Oncotarget. 2016 Mar 29;7(13):16297-310. doi: 10.18632/oncotarget.7484. Oncotarget. 2016. PMID: 26908448 Free PMC article.
-
Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications.Physiol Rev. 2016 Jan;96(1):307-64. doi: 10.1152/physrev.00010.2014. Physiol Rev. 2016. PMID: 26681794 Free PMC article. Review.
-
Reduced EGFR Level in eIF2α PhosphorylationDeficient Hepatocytes Is Responsible for Susceptibility to Oxidative Stress.Mol Cells. 2020 Mar 31;43(3):264-275. doi: 10.14348/molcells.2020.2197. Mol Cells. 2020. PMID: 32150794 Free PMC article.
-
The Role of Mitochondrial and Redox Alterations in the Skeletal Myopathy Associated with Chronic Kidney Disease.Antioxid Redox Signal. 2023 Feb;38(4-6):318-337. doi: 10.1089/ars.2022.0143. Antioxid Redox Signal. 2023. PMID: 36245209 Free PMC article. Review.
-
Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating GAPDH at threonine 237 and decreasing its nuclear translocation.J Biol Chem. 2011 Dec 9;286(49):42211-42220. doi: 10.1074/jbc.M111.296905. Epub 2011 Oct 6. J Biol Chem. 2011. PMID: 21979951 Free PMC article.
References
-
- Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular Regulation by Hydrogen Peroxide. J Am Soc Nephrol. 2003;14:S211–215. - PubMed
-
- Halliwell B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep. 1978;2:113–128. - PubMed
-
- Anderson I, Adinolfi C, Doctrow S, Huffman K, Joy KA, Malfroy B, Soden P, Rupniak HT, Barnes JC. Oxidative signalling and inflammatory pathways in Alzheimer’s disease. Biochem Soc Symp. 2001:141–149. - PubMed
-
- Gandhi S, Wood NW. Molecular pathogenesis of Parkinson’s disease. Hum Mol Genet. 2005;14:2749–2755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038849/NS/NINDS NIH HHS/United States
- R01 NS036778-04/NS/NINDS NIH HHS/United States
- P30 DK092926/DK/NIDDK NIH HHS/United States
- U01 DK076160-01/DK/NIDDK NIH HHS/United States
- R01 NS036778/NS/NINDS NIH HHS/United States
- R01 NS038849-05/NS/NINDS NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- P60 DK020572-27/DK/NIDDK NIH HHS/United States
- NS38849/NS/NINDS NIH HHS/United States
- U01 DK076160/DK/NIDDK NIH HHS/United States
- DK 076160/DK/NIDDK NIH HHS/United States
- R01 NS038849-04/NS/NINDS NIH HHS/United States
- 5P60 DK20572/DK/NIDDK NIH HHS/United States
- NS36778/NS/NINDS NIH HHS/United States
- U01 DK076160-02/DK/NIDDK NIH HHS/United States
- P60 DK020572-26/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

