Expression, purification and in vitro functional reconstitution of the chemokine receptor CCR1
- PMID: 19275940
- PMCID: PMC2706832
- DOI: 10.1016/j.pep.2009.03.001
Expression, purification and in vitro functional reconstitution of the chemokine receptor CCR1
Abstract
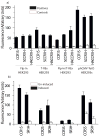
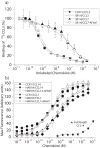
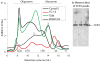
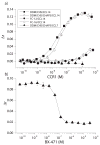
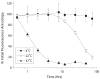
Chemokine receptors are a specific class of G-protein-coupled receptors (GPCRs) that control cell migration associated with routine immune surveillance, inflammation and development. In addition to their roles in normal physiology, these receptors and their ligands are involved in a large number of inflammatory diseases, cancer and AIDS, making them prime therapeutic targets in the pharmaceutical industry. Like other GPCRs, a significant obstacle in determining structures and characterizing mechanisms of activation has been the difficulty in obtaining high levels of pure, functional receptor. Here we describe a systematic effort to express the chemokine receptor CCR1 in mammalian cells, and to purify and reconstitute it in functional form. The highest expression levels were obtained using an inducible HEK293 system. The receptor was purified using a combination of N- (StrepII or Hemagglutinin) and C-terminal (His8) affinity tags. Function was assessed by ligand binding using a novel fluorescence polarization assay with fluorescein-labeled chemokine. A strict dependence of function on the detergent composition was observed, as solubilization of CCR1 in n-dodecyl-beta-D-maltopyranoside/cholesteryl hemisuccinate yielded functional receptor with a K(d) of 21 nM for the chemokine CCL14, whereas it was non-functional in phosphocholine detergents. Differences in function were observed despite the fact that both these detergent types maintained the receptor in a state characterized by monomers and small oligomers, but not large aggregates. While optimization is still warranted, yields of approximately 0.1-0.2mg of pure functional receptor per 10(9) cells will permit biophysical studies of this medically important receptor.
Figures


Similar articles
-
Structural and functional analysis of Ccr1l1, a Rodentia-restricted eosinophil-selective chemokine receptor homologue.J Biol Chem. 2021 Jan-Jun;296:100373. doi: 10.1016/j.jbc.2021.100373. Epub 2021 Feb 3. J Biol Chem. 2021. PMID: 33548230 Free PMC article.
-
Influence of Chemokine N-Terminal Modification on Biased Agonism at the Chemokine Receptor CCR1.Int J Mol Sci. 2019 May 15;20(10):2417. doi: 10.3390/ijms20102417. Int J Mol Sci. 2019. PMID: 31096719 Free PMC article.
-
Evaluation and extension of the two-site, two-step model for binding and activation of the chemokine receptor CCR1.J Biol Chem. 2019 Mar 8;294(10):3464-3475. doi: 10.1074/jbc.RA118.006535. Epub 2018 Dec 19. J Biol Chem. 2019. PMID: 30567735 Free PMC article.
-
Structural Insights into Molecular Recognition and Receptor Activation in Chemokine-Chemokine Receptor Complexes.J Med Chem. 2023 Jun 8;66(11):7070-7085. doi: 10.1021/acs.jmedchem.3c00352. Epub 2023 May 22. J Med Chem. 2023. PMID: 37212620 Review.
-
Chemokine receptor antagonists: Part 1.Expert Opin Ther Pat. 2009 Jan;19(1):39-58. doi: 10.1517/13543770802641346. Expert Opin Ther Pat. 2009. PMID: 19441897 Review.
Cited by
-
NMR in the Analysis of Functional Chemokine Interactions and Drug Discovery.Drug Discov Today Technol. 2012;9(4):e293-e299. doi: 10.1016/j.ddtec.2012.04.003. Drug Discov Today Technol. 2012. PMID: 23166561 Free PMC article.
-
Steroid-Based Amphiphiles for Membrane Protein Study: The Importance of Alkyl Spacers for Protein Stability.Chembiochem. 2018 Jul 4;19(13):1433-1443. doi: 10.1002/cbic.201800106. Epub 2018 May 29. Chembiochem. 2018. PMID: 29660780 Free PMC article.
-
Refolding and characterization of two G protein-coupled receptors purified from E. coli inclusion bodies.PLoS One. 2021 Feb 24;16(2):e0247689. doi: 10.1371/journal.pone.0247689. eCollection 2021. PLoS One. 2021. PMID: 33626080 Free PMC article.
-
Overexpression of membrane proteins in mammalian cells for structural studies.Mol Membr Biol. 2013 Feb;30(1):52-63. doi: 10.3109/09687688.2012.703703. Epub 2012 Sep 11. Mol Membr Biol. 2013. PMID: 22963530 Free PMC article. Review.
-
A rapid and efficient way to obtain modified chemokines for functional and biophysical studies.Cytokine. 2011 Aug;55(2):168-73. doi: 10.1016/j.cyto.2011.05.002. Epub 2011 May 31. Cytokine. 2011. PMID: 21632261 Free PMC article.
References
-
- Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3:530–541. - PubMed
-
- Dalrymple MB, Pfleger KD, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol Ther. 2008;118:359–371. - PubMed
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. - PubMed
-
- Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today. 2006;11:481–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

