Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate LNCaP cells: roles of ERK/MAP kinase
- PMID: 19274665
- PMCID: PMC2753603
- DOI: 10.1002/pros.20944
Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate LNCaP cells: roles of ERK/MAP kinase
Abstract
Background: Accumulated evidence suggests stromal-epithelial interactions are critical to the progression of prostate cancer. In this study, we characterized AR phosphorylation in LNCaP cells co-cultured with the conditioned medium (CM) from human prostate stromal fibroblasts.
Methods: CM harvested from prostate stromal fibroblasts was added to LNCaP cells, and both anchorage-dependent and -independent growth was determined. Status of AR phosphorylation at Ser-81 and Ser-213 was assessed by immunoblot analysis. ERK kinase activity was measured using MBP-2 protein as the substrate.
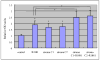
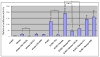
Results: The growth of LNCaP cells on plastic dishes increased by 1.7-fold upon exposure to stromal CM or androgen, and their combination resulted in additive growth (2.4-fold). Anchorage-independent growth of LNCaP cells in soft agar, however, was induced synergistically at 80-fold by both stromal CM and androgen. Stromal CM or androgen alone induced LNCaP cell growth by 10- and 26-fold, respectively. We observed ERK kinase inhibitor, U0126, but not phosphatidylinositol 3-kinase (PI-3K), LY294002, or protein kinase A (PKA) inhibitor, H-89, inhibited stromal CM or androgen-induced PSA promoter luciferase activities, and anchorage-independent growth of LNCaP cells. Our results demonstrated for the first time how stromal CM acts in synergy with androgen by activation of ERK kinase and AR phosphorylation at Ser-81 but not Ser-213, for AR-regulated PSA promoter and anchorage-independent growth of human prostate cancer cells.
Conclusions: A stromal factor-activated ERK pathway mediated by AR phosphorylation at Ser-81 could be responsible for stimulating the growth of human prostate cancer cells.
Figures



Similar articles
-
Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence.Cancer Res. 2004 Oct 1;64(19):7156-68. doi: 10.1158/0008-5472.CAN-04-1121. Cancer Res. 2004. PMID: 15466214
-
Vasoactive intestinal peptide transactivates the androgen receptor through a protein kinase A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells.Mol Pharmacol. 2007 Jul;72(1):73-85. doi: 10.1124/mol.107.033894. Epub 2007 Apr 12. Mol Pharmacol. 2007. PMID: 17430995
-
Androgen-independent prostate cancer DU145 cells suppress androgen-dependent growth of prostate stromal cells through production of inhibitory factors for androgen responsiveness.Biochem Biophys Res Commun. 2003 Jul 4;306(3):629-36. doi: 10.1016/s0006-291x(03)01023-4. Biochem Biophys Res Commun. 2003. PMID: 12810065
-
Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth.Mol Cell Endocrinol. 2002 Nov 29;197(1-2):231-8. doi: 10.1016/s0303-7207(02)00263-0. Mol Cell Endocrinol. 2002. PMID: 12431817 Review.
-
Stromal androgen receptor in prostate development and cancer.Am J Pathol. 2014 Oct;184(10):2598-607. doi: 10.1016/j.ajpath.2014.06.022. Epub 2014 Aug 1. Am J Pathol. 2014. PMID: 25088980 Free PMC article. Review.
Cited by
-
Androgen receptor phosphorylation at serine 515 by Cdk1 predicts biochemical relapse in prostate cancer patients.Br J Cancer. 2013 Jan 15;108(1):139-48. doi: 10.1038/bjc.2012.480. Epub 2012 Dec 4. Br J Cancer. 2013. PMID: 23321516 Free PMC article.
-
Androgen receptor phosphorylation at serine 308 and serine 791 predicts enhanced survival in castrate resistant prostate cancer patients.Int J Mol Sci. 2013 Aug 13;14(8):16656-71. doi: 10.3390/ijms140816656. Int J Mol Sci. 2013. PMID: 23945560 Free PMC article.
-
Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications.Mol Cancer Ther. 2011 May;10(5):720-31. doi: 10.1158/1535-7163.MCT-10-1096. Epub 2011 Mar 10. Mol Cancer Ther. 2011. PMID: 21393425 Free PMC article.
-
Proline-Directed Androgen Receptor Phosphorylation.J Mol Genet Med. 2013 Oct;7(3):75. doi: 10.4172/1747-0862.1000075. J Mol Genet Med. 2013. PMID: 25866551 Free PMC article.
-
Future Aspects of CDK5 in Prostate Cancer: From Pathogenesis to Therapeutic Implications.Int J Mol Sci. 2019 Aug 9;20(16):3881. doi: 10.3390/ijms20163881. Int J Mol Sci. 2019. PMID: 31395805 Free PMC article. Review.
References
-
- Chung LW. Implications of stromal-epithelial interaction in human prostate cancer growth, progression and differentiation. Semin Cancer Biol. 1993;4(3):183–192. - PubMed
-
- Cunha GR, Shannon JM, Neubauer BL, Sawyer LM, Fujii H, Taguchi O, Chung LW. Mesenchymal-epithelial interactions in sex differentiation. Hum Genet. 1981;58(1):68–77. - PubMed
-
- Djakiew D, Pflug B, Onoda M. Stromal-epithelial paracrine interactions in the neoplastic rat and human prostate. Adv Exp Med Biol. 1993;330:185–202. - PubMed
-
- Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70(9-10):506–521. - PubMed
-
- Fu M, Rao M, Wu K, Wang C, Zhang X, Hessien M, Yeung YG, Gioeli D, Weber MJ, Pestell RG. The androgen receptor acetylation site regulates cAMP and AKT but not ERK-induced activity. J Biol Chem. 2004;279(28):29436–29449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

