Alterations of T cell activation signalling and cytokine production by postmenopausal estrogen levels
- PMID: 19265538
- PMCID: PMC2656459
- DOI: 10.1186/1742-4933-6-1
Alterations of T cell activation signalling and cytokine production by postmenopausal estrogen levels
Abstract
Background: Immunosenescence is an age-associated disorder occurring primarily in T cell compartments, including altered subset composition, functions, and activation. In women, evidence implicates diminished estrogen in the postmenopausal period as a contributing factor to diminished T cell responsiveness. Since hypoestrogenism is present in postmenopausal women, our objective focused on whether T cell activation, defined as signalling molecule expressions and activation, and function, identified as IL-2 production, were affected by low estrogen.
Methods: Using Jurkat 6.1 T cells, consequences of 4 pg/ml (corresponding to postmenopausal levels) or 40 pg/ml (premenopausal levels) of estradiol (E(2)) were analyzed on signalling proteins, CD3-zeta, JAK2, and JAK3, determined by Western immunoblotting. These consequences were correlated with corresponding gene expressions, quantified by real time-polymerase chain reaction. Tyrosine phosphorylation of CD3-zeta was defined by immunoprecipitation and western immunoblotting following activation by T cell receptor (TcR) cross-linking. CD3-zeta expression and modulation was also confirmed in T cells from pre- and postmenopausal women. To assess functional consequences, IL-2 production, induced by PMA and ionomycin, was determined using enzyme-linked immunosorbent spot assay (ELISpot).
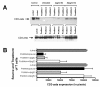
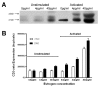
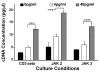
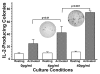
Results: At 40 pg/ml E(2), the level of signalling protein CD3-zeta was elevated 1.57-fold, compared with cells exposed to 4 pg/ml E(2). The CD3-zeta proteins also exhibited altered levels of activation-induced phosphorylation in the presence of 40 pg/ml E(2) versus 4 pg/ml: 23 kD phosphorylated form increased 2.64-fold and the 21 kD form was elevated 2.95-fold. Examination of kinases associated with activation signalling also demonstrated that, in the presence of 40 pg/ml E(2), JAK2 protein expression was increased 1.64-fold (p < 0.001) and JAK3 enhanced 1.79-fold (p < 0.001) compared to 4 pg/ml. mRNA levels for CD3-zeta, JAK2, and JAK3 were significantly increased following exposure to 40 pg/ml E(2) (2.39, 2.01, and 2.21 fold, respectively) versus 4 pg/ml. These findings were confirmed in vivo, since T cells from postmenopausal women exhibited 7.2-fold diminished CD3-zeta expression, compared to pre-menopausal controls and this expression was elevated 3.8-fold by addition of 40 pg/ml E(2). Functionally, Jurkat cells exposed to 40 pg/ml E(2) and activated exhibited significantly elevated numbers of IL-2 producing colonies compared to 4 pg/ml (75.3 +/- 2.2 versus 55.7 +/- 2.1 colonies, p < 0.0001).
Conclusion: Jurkat T cells exposed to 4 pg/ml E(2) expressed significantly diminished activation signalling proteins, correlating with reduced IL-2 production. Lower signalling protein levels appear to result from decreased CD3-zeta, JAK2, and JAK3 gene expressions. These findings may provide a molecular basis for immunosenescence associated with the postmenopausal state.
Figures



Similar articles
-
Genistein reverses diminished T-cell signal transduction, induced by post-menopausal estrogen levels.Am J Reprod Immunol. 2009 Jan;61(1):26-33. doi: 10.1111/j.1600-0897.2008.00658.x. Am J Reprod Immunol. 2009. PMID: 19086989
-
MHC class I ligation of human T cells activates the ZAP70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 zeta-chain, and induces apoptosis.J Immunol. 1997 Apr 1;158(7):3189-96. J Immunol. 1997. PMID: 9120273
-
CD3ζ-chain expression of human T lymphocytes is regulated by TNF via Src-like adaptor protein-dependent proteasomal degradation.J Immunol. 2012 Aug 15;189(4):1602-10. doi: 10.4049/jimmunol.1102365. Epub 2012 Jul 13. J Immunol. 2012. PMID: 22798681
-
Modulatory effect of aggregating the CD3 molecular complex on T cell activation.Cell Immunol. 1992 Mar;140(1):81-96. doi: 10.1016/0008-8749(92)90178-r. Cell Immunol. 1992. PMID: 1531456
-
Ras initiates phosphatidyl-inositol-3-kinase (PI3K)/PKB mediated signalling pathways in untransformed human peripheral blood T lymphocytes.Adv Enzyme Regul. 2005;45:52-62. doi: 10.1016/j.advenzreg.2005.02.005. Epub 2005 Aug 9. Adv Enzyme Regul. 2005. PMID: 16083947 Review.
Cited by
-
Ovarian aging is associated with gray matter volume and disability in women with MS.Neurology. 2018 Jan 16;90(3):e254-e260. doi: 10.1212/WNL.0000000000004843. Epub 2017 Dec 22. Neurology. 2018. PMID: 29273686 Free PMC article.
-
Effect of Chinese herbal medicines for nourishing yin, supplementing qi, and activating blood on reproductive endocrine activity and immune functions in patients with primary Sjogren's syndrome.Chin J Integr Med. 2015 Oct;21(10):778-83. doi: 10.1007/s11655-015-2323-y. Epub 2015 Nov 3. Chin J Integr Med. 2015. PMID: 26525549
-
Phytoestrogen-Based Hormonal Replacement Therapy Could Benefit Women Suffering Late-Onset Asthma.Int J Mol Sci. 2023 Oct 19;24(20):15335. doi: 10.3390/ijms242015335. Int J Mol Sci. 2023. PMID: 37895016 Free PMC article. Review.
References
-
- Franceschi C, Bonafè M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. - PubMed
-
- Linton PJ, Lustgarten J, Thoman M. T cell function in the aged: Lessons learned from animal models. Clin Appl Immunol Rev. 2006;6:73–97.
-
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. - PubMed
-
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. - PubMed
-
- Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ, Adler WH. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest. 1988;81:1096–1102. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

