The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer
- PMID: 19259277
- PMCID: PMC2632563
The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer
Abstract
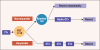
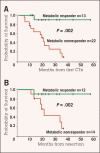
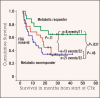
Preoperative induction therapy in stages II and III adenocarcinoma of the esophagogastric junction (AEG) and gastric cancer is now an accepted treatment choice in the Western world. Patients who respond to induction therapy have significantly improved survival compared to nonresponding patients. Until recently, however, no prospectively tested markers for predicting response and/or prognosis in this settingwere available. The MUNICON I study recently showed the utility of fluorodeoxyglucose-positron emission tomography (FDG-PET) in predicting response and prognosis in AEG and indicated the feasibility of a PET-guided treatment algorithm. These findings are an important step forward in tailoring multimodal treatment to tumor biology. In gastric cancer, the issue is more complicated, because approximately 30% of these cancers cannot be visualized with sufficient contrast for quantification. Insufficient FDG uptake is mostly associated with diffusetype gastric cancer with signet cells and mucinous content. In FDG avid patients, FDG-PET can be used for response evaluation. The prognosis of nonavid patients is similar to metabolic nonresponders. The addition of new tracers (eg, fluorothymidine) might increase the accuracy of these tests in the future. In AEG types I and II, PET-guided induction therapy is feasible and will undergo further evaluation in a randomized multicenter trial. In gastric cancer, there should be consideration of such treatment concepts as immediate resection after 2 weeks of induction therapy with or without adjuvant treatment in metabolic nonresponders or modified chemotherapy regimens possibly including biologically targeted drugs in FDG non-avid tumors.
Figures

Similar articles
-
The new credo: induction chemotherapy in locally advanced gastric cancer: consequences for surgical strategies.Gastric Cancer. 2008;11(1):1-9. doi: 10.1007/s10120-007-0448-1. Epub 2008 Mar 29. Gastric Cancer. 2008. PMID: 18373171 Review.
-
(18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial.J Nucl Med. 2011 Aug;52(8):1189-96. doi: 10.2967/jnumed.110.085803. Epub 2011 Jul 15. J Nucl Med. 2011. PMID: 21764790 Clinical Trial.
-
Optimizing neoadjuvant chemotherapy through the use of early response evaluation by positron emission tomography.Recent Results Cancer Res. 2012;196:201-11. doi: 10.1007/978-3-642-31629-6_14. Recent Results Cancer Res. 2012. PMID: 23129376 Review.
-
PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial.Lancet Oncol. 2007 Sep;8(9):797-805. doi: 10.1016/S1470-2045(07)70244-9. Lancet Oncol. 2007. PMID: 17693134 Clinical Trial.
-
Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study.Clin Cancer Res. 2008 Apr 1;14(7):2012-8. doi: 10.1158/1078-0432.CCR-07-0934. Clin Cancer Res. 2008. PMID: 18381939
Cited by
-
Approaches and genetic determinants in predicting response to neoadjuvant chemotherapy in locally advanced gastric cancer.Oncotarget. 2017 May 2;8(18):30477-30494. doi: 10.18632/oncotarget.12955. Oncotarget. 2017. PMID: 27802185 Free PMC article. Review.
-
Development and Validation of a Computed Tomography-Based Radiomics Signature to Predict Response to Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer.JAMA Netw Open. 2021 Aug 2;4(8):e2121143. doi: 10.1001/jamanetworkopen.2021.21143. JAMA Netw Open. 2021. PMID: 34410397 Free PMC article.
-
Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update.Nagoya J Med Sci. 2017 Nov;79(4):527-543. doi: 10.18999/nagjms.79.4.527. Nagoya J Med Sci. 2017. PMID: 29238109 Free PMC article. Review.
-
Pharmacokinetics, metabolism, biodistribution, radiation dosimetry, and toxicology of (18)F-fluoroacetate ((18)F-FACE) in non-human primates.Mol Imaging Biol. 2012 Apr;14(2):213-24. doi: 10.1007/s11307-011-0485-3. Mol Imaging Biol. 2012. PMID: 21437735 Free PMC article.
-
Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma.Br J Surg. 2017 Dec;104(13):1816-1828. doi: 10.1002/bjs.10627. Epub 2017 Sep 25. Br J Surg. 2017. PMID: 28944954 Free PMC article.
References
-
- Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-Fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25(suppl) abstract 4510.
-
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. - PubMed
-
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. - PubMed
-
- Heidecke CD, Weighardt H, Feith M, et al. Neoadjuvant treatment of esophageal cancer: immunosuppression following combined radiochemotherapy. Surgery. 2002;132:495–501. - PubMed
LinkOut - more resources
Full Text Sources
