Preclinical transfusion-dependent humanized mouse model of beta thalassemia major
- PMID: 19258591
- PMCID: PMC2680376
- DOI: 10.1182/blood-2008-12-197012
Preclinical transfusion-dependent humanized mouse model of beta thalassemia major
Abstract
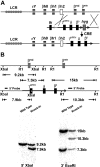
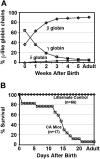
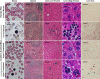
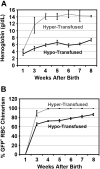
A preclinical humanized mouse model of beta thalassemia major or Cooley anemia (CA) was generated by targeted gene replacement of the mouse adult globin genes in embryonic stem cells. The mouse adult alpha and beta globin genes were replaced with adult human alpha globin genes (alpha2alpha1) and a human fetal to adult hemoglobin (Hb)-switching cassette (gamma(HPFH)deltabeta(0)), respectively. Similar to human infants with CA, fully humanized mice survived postnatally by synthesizing predominantly human fetal Hb, HbF (alpha(2)gamma(2)), with a small amount of human minor adult Hb, HbA2 (alpha(2)delta(2)). Completion of the human fetal to adult Hb switch after birth resulted in severe anemia marked by erythroid hyperplasia, ineffective erythropoiesis, hemolysis, and death. Similar to human patients, CA mice were rescued from lethal anemia by regular blood transfusion. Transfusion corrected the anemia and effectively suppressed the ineffective erythropoiesis, but led to iron overload. This preclinical humanized animal model of CA will be useful for the development of new transfusion and iron chelation regimens, the study of iron homeostasis in disease, and testing of cellular and genetic therapies for the correction of thalassemia.
Figures


Similar articles
-
A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer.Blood. 2003 Apr 15;101(8):2932-9. doi: 10.1182/blood-2002-10-3305. Epub 2002 Dec 12. Blood. 2003. PMID: 12480689
-
Humanized mouse models of Cooley's anemia: correct fetal-to-adult hemoglobin switching, disease onset, and disease pathology.Ann N Y Acad Sci. 2010 Aug;1202:45-51. doi: 10.1111/j.1749-6632.2010.05547.x. Ann N Y Acad Sci. 2010. PMID: 20712771 Free PMC article.
-
Functional requirements for phenotypic correction of murine beta-thalassemia: implications for human gene therapy.Blood. 2001 May 15;97(10):3275-82. doi: 10.1182/blood.v97.10.3275. Blood. 2001. PMID: 11342459
-
Successful correction of the human Cooley's anemia beta-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator.Ann N Y Acad Sci. 2005;1054:238-49. doi: 10.1196/annals.1345.030. Ann N Y Acad Sci. 2005. PMID: 16339671 Review.
-
New therapeutic targets in transfusion-dependent and -independent thalassemia.Hematology Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):278-283. doi: 10.1182/asheducation-2017.1.278. Hematology Am Soc Hematol Educ Program. 2017. PMID: 29222267 Free PMC article. Review.
Cited by
-
Erythropoiesis in the absence of adult hemoglobin.Mol Cell Biol. 2013 Jun;33(11):2241-51. doi: 10.1128/MCB.01734-12. Epub 2013 Mar 25. Mol Cell Biol. 2013. PMID: 23530053 Free PMC article.
-
Generation of an immortalised erythroid cell line from haematopoietic stem cells of a haemoglobin E/β-thalassemia patient.Sci Rep. 2020 Oct 8;10(1):16798. doi: 10.1038/s41598-020-73991-4. Sci Rep. 2020. PMID: 33033327 Free PMC article.
-
Human globin knock-in mice complete fetal-to-adult hemoglobin switching in postnatal development.Mol Cell Biol. 2011 Feb;31(4):876-83. doi: 10.1128/MCB.00725-10. Epub 2010 Dec 20. Mol Cell Biol. 2011. PMID: 21173165 Free PMC article.
-
Recent trends in the gene therapy of β-thalassemia.J Blood Med. 2015 Feb 19;6:69-85. doi: 10.2147/JBM.S46256. eCollection 2015. J Blood Med. 2015. PMID: 25737641 Free PMC article. Review.
-
Humanising the mouse genome piece by piece.Nat Commun. 2019 Apr 23;10(1):1845. doi: 10.1038/s41467-019-09716-7. Nat Commun. 2019. PMID: 31015419 Free PMC article. Review.
References
-
- Mathias LA, Fisher TC, Zeng L, et al. Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol. 2000;28:1343–1353. - PubMed
-
- Centis F, Tabellini L, Lucarelli G, et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with β-thalassemia major. Blood. 2000;96:3624–3629. - PubMed
-
- Yuan J, Angelucci E, Lucarelli G, et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe β-thalassemia (Cooley's anemia). Blood. 1993;82:374–377. - PubMed
-
- Cazzola M, Alessandrino P, Barosi G, Morandi S, Stefanelli M. Quantitative evaluation of the mechanisms of the anaemia in heterozygous β-thalassaemia. Scand J Haematol. 1979;23:107–114. - PubMed
-
- Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL. Comparison of mechanisms of anemia in mice with sickle cell disease and β-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol. 2002;30:394–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

