A Notch updated
- PMID: 19255248
- PMCID: PMC2686403
- DOI: 10.1083/jcb.200811141
A Notch updated
Abstract
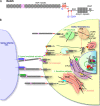
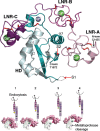
Cell-cell signaling mediated by the Notch receptor is iteratively involved in numerous developmental contexts, and its dysregulation has been associated with inherited genetic disorders and cancers. The core components of the signaling pathway have been identified for some time, but the study of the modulation of the pathway in different cellular contexts has revealed many layers of regulation. These include complex sugar modifications in the extracellular domain as well as transit of Notch through defined cellular compartments, including specific endosomes.
Figures
Similar articles
-
Endocytosis-independent mechanisms of Delta ligand proteolysis.Exp Cell Res. 2006 May 1;312(8):1345-60. doi: 10.1016/j.yexcr.2005.12.037. Epub 2006 Feb 17. Exp Cell Res. 2006. PMID: 16487968
-
Structural and functional characterization of the NHR1 domain of the Drosophila neuralized E3 ligase in the notch signaling pathway.J Mol Biol. 2009 Oct 23;393(2):478-95. doi: 10.1016/j.jmb.2009.08.020. Epub 2009 Aug 14. J Mol Biol. 2009. PMID: 19683535
-
A role of receptor Notch in ligand cis-inhibition in Drosophila.Curr Biol. 2010 Mar 23;20(6):554-60. doi: 10.1016/j.cub.2010.01.058. Epub 2010 Mar 11. Curr Biol. 2010. PMID: 20226663
-
Notch signaling and Notch signaling modifiers.Int J Biochem Cell Biol. 2011 Nov;43(11):1550-62. doi: 10.1016/j.biocel.2011.08.005. Epub 2011 Aug 12. Int J Biochem Cell Biol. 2011. PMID: 21854867 Free PMC article. Review.
-
Structural Insights into Notch Receptor-Ligand Interactions.Adv Exp Med Biol. 2018;1066:33-46. doi: 10.1007/978-3-319-89512-3_2. Adv Exp Med Biol. 2018. PMID: 30030820 Review.
Cited by
-
Notch1 contributes to chemoresistance to gemcitabine and serves as an unfavorable prognostic indicator in pancreatic cancer.World J Surg. 2013 Jul;37(7):1688-94. doi: 10.1007/s00268-013-2010-0. World J Surg. 2013. PMID: 23568245
-
Numb Suppresses Notch-Dependent Activation of Enhancer of split during Lateral Inhibition in the Drosophila Embryonic Nervous System.Biomolecules. 2024 Aug 26;14(9):1062. doi: 10.3390/biom14091062. Biomolecules. 2024. PMID: 39334829 Free PMC article.
-
The very many faces of presenilins and the γ-secretase complex.Protoplasma. 2013 Oct;250(5):997-1011. doi: 10.1007/s00709-013-0494-y. Epub 2013 Mar 16. Protoplasma. 2013. PMID: 23504135 Free PMC article. Review.
-
Analysis of endogenous NOTCH1 from POFUT1 S162L patient fibroblasts reveals the importance of the O-fucose modification on EGF12 in human development.Glycobiology. 2024 Jun 22;34(8):cwae047. doi: 10.1093/glycob/cwae047. Glycobiology. 2024. PMID: 38976017
-
Rabconnectin-3 is a functional regulator of mammalian Notch signaling.J Biol Chem. 2010 Nov 5;285(45):34757-64. doi: 10.1074/jbc.M110.158634. Epub 2010 Sep 1. J Biol Chem. 2010. PMID: 20810660 Free PMC article.
References
-
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. 1999. Notch signaling: cell fate control and signal integration in development.Science. 284:770–776 - PubMed
-
- Baba T., Damke H., Hinshaw J.E., Ikeda K., Schmid S.L., Warnock D.E. 1995. Role of dynamin in clathrin-coated vesicle formation.Cold Spring Harb. Symp. Quant. Biol. 60:235–242 - PubMed
-
- Boldt H.B., Kjaer-Sorensen K., Overgaard M.T., Weyer K., Poulsen C.B., Sottrup-Jensen L., Conover C.A., Giudice L.C., Oxvig C. 2004. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity.J. Biol. Chem. 279:38525–38531 - PubMed
-
- Bray S.J. 2006. Notch signalling: a simple pathway becomes complex.Nat. Rev. Mol. Cell Biol. 7:678–689 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

