Retinal insulin receptor signaling in hyperosmotic stress
- PMID: 19251051
- PMCID: PMC2670484
- DOI: 10.1016/S0083-6729(08)00620-1
Retinal insulin receptor signaling in hyperosmotic stress
Abstract
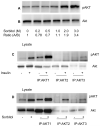
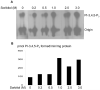
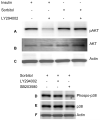
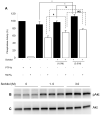
In the diabetic eye, the increased accumulation of sorbitol in the retina has been implicated in the pathogenesis of diabetic retinopathy (DR). Neurodegeneration is an important component of DR as demonstrated by increased neural apoptosis in the retina during experimental and human diabetes. Insulin receptor (IR) activation has been shown to rescue retinal neurons from apoptosis through a phosphoinositide 3-kinase and protein kinase B (Akt) survival cascade. In this study, we examined the IR signaling in sorbitol-induced hyperosmotic stressed retinas.
Figures







Similar articles
-
PDGF- and insulin/IGF-1-specific distinct modes of class IA PI 3-kinase activation in normal rat retinas and RGC-5 retinal ganglion cells.Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3687-98. doi: 10.1167/iovs.07-1455. Epub 2008 Apr 17. Invest Ophthalmol Vis Sci. 2008. PMID: 18421086
-
Insulin growth factor 1 receptor/PI3K/AKT survival pathway in outer segment membranes of rod photoreceptors.Invest Ophthalmol Vis Sci. 2008 Nov;49(11):4765-73. doi: 10.1167/iovs.08-2286. Epub 2008 Jun 19. Invest Ophthalmol Vis Sci. 2008. PMID: 18566464 Free PMC article.
-
Interaction of the retinal insulin receptor beta-subunit with the p85 subunit of phosphoinositide 3-kinase.Biochemistry. 2004 May 18;43(19):5637-50. doi: 10.1021/bi035913v. Biochemistry. 2004. PMID: 15134438
-
Characterization of insulin signaling in rat retina in vivo and ex vivo.Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E763-74. doi: 10.1152/ajpendo.00507.2002. Epub 2003 Jun 10. Am J Physiol Endocrinol Metab. 2003. PMID: 12799319
-
Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol.J Biol Chem. 2003 Oct 3;278(40):38484-94. doi: 10.1074/jbc.M211824200. Epub 2003 Jul 24. J Biol Chem. 2003. PMID: 12881532
Cited by
-
Phosphoinositide 3-kinase signaling in the vertebrate retina.J Lipid Res. 2010 Jan;51(1):4-22. doi: 10.1194/jlr.R000232. J Lipid Res. 2010. PMID: 19638643 Free PMC article. Review.
-
Activation of sorbitol pathway in metabolic syndrome and increased susceptibility to cataract in Wistar-Obese rats.Mol Vis. 2012;18:495-503. Epub 2012 Feb 24. Mol Vis. 2012. PMID: 22393276 Free PMC article.
-
The role of insulin-like growth factor-I in the physiopathology of hearing.Front Mol Neurosci. 2011 Jul 25;4:11. doi: 10.3389/fnmol.2011.00011. eCollection 2011. Front Mol Neurosci. 2011. PMID: 21845174 Free PMC article.
-
Reducing Akt2 in retinal pigment epithelial cells causes a compensatory increase in Akt1 and attenuates diabetic retinopathy.Nat Commun. 2022 Oct 13;13(1):6045. doi: 10.1038/s41467-022-33773-0. Nat Commun. 2022. PMID: 36229454 Free PMC article.
-
Nrn1 Overexpression Attenuates Retinal Ganglion Cell Apoptosis, Promotes Axonal Regeneration, and Improves Visual Function Following Optic Nerve Crush in Rats.J Mol Neurosci. 2021 Jan;71(1):66-79. doi: 10.1007/s12031-020-01627-3. Epub 2020 Jun 30. J Mol Neurosci. 2021. PMID: 32607759
References
-
- Ahmed Z, Smith BJ, Pillay TS. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 2000;475:31–34. - PubMed
-
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511. - PubMed
-
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821. - PubMed
-
- Barnett PA, Gonzalez RG, Chylack LT, Jr., Cheng HM. The effect of oxidation on sorbitol pathway kinetics. Diabetes. 1986;35:426–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

