The sensor kinase TodS operates by a multiple step phosphorelay mechanism involving two autokinase domains
- PMID: 19240030
- PMCID: PMC2667722
- DOI: 10.1074/jbc.M900521200
The sensor kinase TodS operates by a multiple step phosphorelay mechanism involving two autokinase domains
Abstract
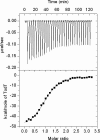
Expression of the Pseudomonas putida tod operon, which encodes enzymes for toluene metabolism, takes place from the P(todX) promoter and is mediated by the TodS/TodT two component system. The sensor kinase TodS has a complex domain arrangement containing two functional modules, each harboring a sensor- and an autokinase domain and separated by a receiver domain. Based on site-directed mutagenesis of phosphoaccepting His-190, Asp-500, and His-760 and in vitro transphosphorylation experiments with recombinant TodS fragments, we show that TodS uses a multiple step phosphorelay mechanism to activate TodT. Toluene binding stimulates exclusively phosphorylation of His-190, which is followed by phosphotransfer to Asp-500 and subsequently to His-760 prior to phosphorylation of TodT Asp-57. Mutation of His-190, Asp-500, and H760A prevented up-regulation of toluene-mediated stimulation of TodT transphosphorylation in vitro and reduced in vivo expression of P(todX) to the basal level. Calorimetric studies support that TodT binds to the C-terminal kinase module with a K(D) of approximately 200 nm and 1:1 stoichiometry. This is the first report of a multiple step phosphorelay mechanism of a sensor kinase that involves two autokinase domains.
Figures





Similar articles
-
Construction of a prototype two-component system from the phosphorelay system TodS/TodT.Protein Eng Des Sel. 2012 Apr;25(4):159-69. doi: 10.1093/protein/gzs001. Epub 2012 Feb 3. Protein Eng Des Sel. 2012. PMID: 22308529
-
Catabolite repression of the TodS/TodT two-component system and effector-dependent transphosphorylation of TodT as the basis for toluene dioxygenase catabolic pathway control.J Bacteriol. 2010 Aug;192(16):4246-50. doi: 10.1128/JB.00379-10. Epub 2010 Jun 11. J Bacteriol. 2010. PMID: 20543072 Free PMC article.
-
Hierarchical binding of the TodT response regulator to its multiple recognition sites at the tod pathway operon promoter.J Mol Biol. 2008 Feb 15;376(2):325-37. doi: 10.1016/j.jmb.2007.12.004. Epub 2007 Dec 8. J Mol Biol. 2008. PMID: 18166197
-
His-Asp phosphorelay signaling: a communication avenue between plants and their environment.Plant Mol Biol. 2000 Jan;42(2):273-8. doi: 10.1023/a:1006334926388. Plant Mol Biol. 2000. PMID: 10794527 Review.
-
Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid-encoded regulators.Annu Rev Microbiol. 1997;51:341-73. doi: 10.1146/annurev.micro.51.1.341. Annu Rev Microbiol. 1997. PMID: 9343354 Review.
Cited by
-
Genetic Analysis Reveals a Requirement for the Hybrid Sensor Kinase RscS in para-Aminobenzoic Acid/Calcium-Induced Biofilm Formation by Vibrio fischeri.J Bacteriol. 2023 Jul 25;205(7):e0007523. doi: 10.1128/jb.00075-23. Epub 2023 Jun 12. J Bacteriol. 2023. PMID: 37306594 Free PMC article.
-
Fluoro-recognition: New in vivo fluorescent assay for toluene dioxygenase probing induction by and metabolism of polyfluorinated compounds.Environ Microbiol. 2022 Nov;24(11):5202-5216. doi: 10.1111/1462-2920.16187. Epub 2022 Oct 17. Environ Microbiol. 2022. PMID: 36054238 Free PMC article.
-
Pseudomonas aeruginosa as a Model To Study Chemosensory Pathway Signaling.Microbiol Mol Biol Rev. 2021 Jan 13;85(1):e00151-20. doi: 10.1128/MMBR.00151-20. Print 2021 Feb 17. Microbiol Mol Biol Rev. 2021. PMID: 33441490 Free PMC article. Review.
-
Transcriptional Regulation of the Peripheral Pathway for the Anaerobic Catabolism of Toluene and m-Xylene in Azoarcus sp. CIB.Front Microbiol. 2018 Mar 22;9:506. doi: 10.3389/fmicb.2018.00506. eCollection 2018. Front Microbiol. 2018. PMID: 29623071 Free PMC article.
-
New family of biosensors for monitoring BTX in aquatic and edaphic environments.Microb Biotechnol. 2016 Nov;9(6):858-867. doi: 10.1111/1751-7915.12394. Epub 2016 Aug 3. Microb Biotechnol. 2016. PMID: 27484951 Free PMC article.
References
-
- Stock, A. M., Robinson, V. L., and Goudreau, P. N. (2000) Annu. Rev. Biochem. 69 183–215 - PubMed
-
- Appleby, J. L., Parkinson, J. S., and Bourret, R. B. (1996) Cell 86 845–848 - PubMed
-
- Zhang, W., and Shi, L. (2005) Microbiology 151 2159–2173 - PubMed
-
- Varughese, K. I., Madhusudan Zhou, X. Z., Whiteley, J. M., and Hoch, J. A. (1998) Mol. Cell. 2 485–493 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

