Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta
- PMID: 19238383
- PMCID: PMC11030051
- DOI: 10.1007/s00262-009-0667-x
Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta
Abstract
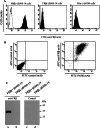
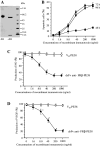
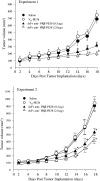
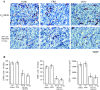
Tumor-associated macrophages (TAMs) are frequently found in glioblastomas and a high degree of macrophage infiltration is associated with a poor prognosis for glioblastoma patients. However, it is unclear whether TAMs in glioblastomas promote tumor growth. In this study, we found that folate receptor beta (FR beta) was expressed on macrophages in human glioblastomas and a rat C6 glioma implanted subcutaneously in nude mice. To target FR beta-expressing TAMs, we produced a recombinant immunotoxin consisting of immunoglobulin heavy and light chain Fv portions of an anti-mouse FR beta monoclonal antibody and Pseudomonas exotoxin A. Injection of the immunotoxin into C6 glioma xenografts in nude mice significantly depleted TAMs and reduced tumor growth. The immunotoxin targeting FR beta-expressing macrophages will provide a therapeutic tool for human glioblastomas.
Figures

Similar articles
-
Effect of an immunotoxin to folate receptor beta on bleomycin-induced experimental pulmonary fibrosis.Clin Exp Immunol. 2010 Aug;161(2):348-56. doi: 10.1111/j.1365-2249.2010.04182.x. Epub 2010 Jun 9. Clin Exp Immunol. 2010. PMID: 20550546 Free PMC article.
-
Effectiveness of anti-folate receptor beta antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages.Arthritis Rheum. 2005 Sep;52(9):2666-75. doi: 10.1002/art.21228. Arthritis Rheum. 2005. PMID: 16142741
-
In vivo imaging of immunotoxin treatment using Katushka-transfected A-431 cells in a murine xenograft tumour model.Cancer Immunol Immunother. 2012 Oct;61(10):1617-26. doi: 10.1007/s00262-012-1219-3. Epub 2012 Feb 19. Cancer Immunol Immunother. 2012. PMID: 22350071 Free PMC article.
-
Immunotoxins in the treatment of refractory hairy cell leukemia.Hematol Oncol Clin North Am. 2006 Oct;20(5):1137-51, viii. doi: 10.1016/j.hoc.2006.06.009. Hematol Oncol Clin North Am. 2006. PMID: 16990113 Review.
-
Cintredekin besudotox in treatment of malignant glioma.Expert Opin Biol Ther. 2008 Jun;8(6):805-12. doi: 10.1517/14712598.8.6.805. Expert Opin Biol Ther. 2008. PMID: 18476792 Review.
Cited by
-
Novel Therapy for Atherosclerosis Using Recombinant Immunotoxin Against Folate Receptor β-Expressing Macrophages.J Am Heart Assoc. 2012 Aug;1(4):e003079. doi: 10.1161/JAHA.112.003079. Epub 2012 Aug 24. J Am Heart Assoc. 2012. PMID: 23130174 Free PMC article.
-
Effect of an immunotoxin to folate receptor beta on bleomycin-induced experimental pulmonary fibrosis.Clin Exp Immunol. 2010 Aug;161(2):348-56. doi: 10.1111/j.1365-2249.2010.04182.x. Epub 2010 Jun 9. Clin Exp Immunol. 2010. PMID: 20550546 Free PMC article.
-
Folate Transport and One-Carbon Metabolism in Targeted Therapies of Epithelial Ovarian Cancer.Cancers (Basel). 2021 Dec 31;14(1):191. doi: 10.3390/cancers14010191. Cancers (Basel). 2021. PMID: 35008360 Free PMC article. Review.
-
Glioblastoma-Derived IL6 Induces Immunosuppressive Peripheral Myeloid Cell PD-L1 and Promotes Tumor Growth.Clin Cancer Res. 2019 Jun 15;25(12):3643-3657. doi: 10.1158/1078-0432.CCR-18-2402. Epub 2019 Mar 1. Clin Cancer Res. 2019. PMID: 30824583 Free PMC article.
-
Tumor-Associated Macrophages: Combination of Therapies, the Approach to Improve Cancer Treatment.Int J Mol Sci. 2021 Jul 5;22(13):7239. doi: 10.3390/ijms22137239. Int J Mol Sci. 2021. PMID: 34281293 Free PMC article. Review.
References
-
- Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. - DOI - PMC - PubMed
-
- Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res. 2006;25:365–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

