FMR1: a gene with three faces
- PMID: 19233246
- PMCID: PMC2692361
- DOI: 10.1016/j.bbagen.2009.02.007
FMR1: a gene with three faces
Abstract
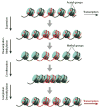
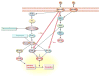
The FMR1 gene is involved in three different syndromes, the fragile X syndrome (FXS), premature ovarian insufficiency (POI) and the fragile X-associated tremor/ataxia syndrome (FXTAS) at older age. Fragile X syndrome is caused by an expansion of a CGG repeat above 200 units in the FMR1 gene resulting in the absence of the FMR1 mRNA and protein. The FMR1 protein is proposed to act as a regulator of mRNA transport and of translation of target mRNAs at the synapse. FXS is seen as a loss of function disorder. POI and FXTAS are found in individuals with an expanded repeat between 50 and 200 CGGs and are associated with increased FMR1 mRNA levels. The presence of elevated FMR1 mRNA in FXTAS suggests that FXTAS may represent a toxic RNA gain-of-function effect. The molecular basis of POI is yet unknown. The role of the FMR1 gene in these disorders is discussed.
Figures

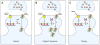
Similar articles
-
Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency.Hum Reprod. 2016 Jan;31(1):158-68. doi: 10.1093/humrep/dev280. Epub 2015 Nov 3. Hum Reprod. 2016. PMID: 26537920 Free PMC article.
-
Unstable mutations in the FMR1 gene and the phenotypes.Adv Exp Med Biol. 2012;769:78-114. doi: 10.1007/978-1-4614-5434-2_6. Adv Exp Med Biol. 2012. PMID: 23560306 Free PMC article. Review.
-
[FMR1 PREMUTATION CARRIERS - ARE THEY REALLY ASYMPTOMATIC?].Harefuah. 2018 Apr;157(4):241-244. Harefuah. 2018. PMID: 29688643 Review. Hebrew.
-
FMRP ribonucleoprotein complexes and RNA homeostasis.Adv Genet. 2020;105:95-136. doi: 10.1016/bs.adgen.2020.01.001. Epub 2020 Feb 6. Adv Genet. 2020. PMID: 32560791 Review.
-
A fragile balance: FMR1 expression levels.Hum Mol Genet. 2003 Oct 15;12 Spec No 2:R249-57. doi: 10.1093/hmg/ddg298. Epub 2003 Sep 2. Hum Mol Genet. 2003. PMID: 12952862 Review.
Cited by
-
The effects of prenatal H1N1 infection at E16 on FMRP, glutamate, GABA, and reelin signaling systems in developing murine cerebellum.J Neurosci Res. 2017 May;95(5):1110-1122. doi: 10.1002/jnr.23949. Epub 2016 Oct 13. J Neurosci Res. 2017. PMID: 27735078 Free PMC article.
-
Transmission of an FMR1 premutation allele in a large family identified through newborn screening: the role of AGG interruptions.J Hum Genet. 2013 Aug;58(8):553-9. doi: 10.1038/jhg.2013.50. Epub 2013 Jun 6. J Hum Genet. 2013. PMID: 23739124 Free PMC article.
-
The protection of acetylcholinesterase inhibitor on β-amyloid-induced the injury of neurite outgrowth via regulating axon guidance related genes expression in neuronal cells.Int J Clin Exp Pathol. 2012;5(9):900-13. Epub 2012 Oct 20. Int J Clin Exp Pathol. 2012. PMID: 23119107 Free PMC article.
-
Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis.Neuron. 2013 Aug 7;79(3):416-38. doi: 10.1016/j.neuron.2013.07.033. Neuron. 2013. PMID: 23931993 Free PMC article. Review.
-
MS-MLPA analysis for FMR1 gene: evaluation in a routine diagnostic setting.BMC Med Genet. 2013 Aug 5;14:79. doi: 10.1186/1471-2350-14-79. BMC Med Genet. 2013. PMID: 23914933 Free PMC article.
References
-
- Hagerman RJ. The physical and behavioural phenotype. In: Hagerman RJ, Hagerman P, editors. Fragile-X syndrome: diagnosis, treatment and research. The Johns Hopkins University Press; Baltimore: 2002. pp. 3–109.
-
- Sherman SL, Jacobs PA, Morton NE, Froster-Iskenius U, Howard-Peebles PN, Nielsen KB, Partington MW, Sutherland GR, Turner G, Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69:289–299. - PubMed
-
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, Van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. - PubMed
-
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick R, Jr, Warren ST, Oostra BA, Nelson DL, Caskey CT. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. - PubMed
-
- Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

