BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation
- PMID: 19224984
- PMCID: PMC2668702
- DOI: 10.1242/dev.029926
BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation
Abstract
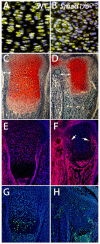
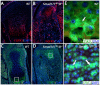
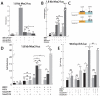
Bone morphogenetic protein (BMP) signaling is required for endochondral bone formation. However, whether or not the effects of BMPs are mediated via canonical Smad pathways or through noncanonical pathways is unknown. In this study we have determined the role of receptor Smads 1, 5 and 8 in chondrogenesis. Deletion of individual Smads results in viable and fertile mice. Combined loss of Smads 1, 5 and 8, however, results in severe chondrodysplasia. Smad1/5(CKO) (cartilage-specific knockout) mutant mice are nearly identical to Smad1/5(CKO);Smad8(-/-) mutants, indicating that Smads 1 and 5 have overlapping functions and are more important than Smad8 in cartilage. The Smad1/5(CKO) phenotype is more severe than that of Smad4(CKO) mice, challenging the dogma, at least in chondrocytes, that Smad4 is required to mediate Smad signaling through BMP pathways. The chondrodysplasia in Smad1/5(CKO) mice is accompanied by imbalances in cross-talk between the BMP, FGF and Ihh/PTHrP pathways. We show that Ihh is a direct target of BMP pathways in chondrocytes, and that FGF exerts antagonistic effects on Ihh expression. Finally, we tested whether FGF exerts its antagonistic effects directly through Smad linker phosphorylation. The results support the alternative conclusion that the effects of FGFs on BMP signaling are indirect in vivo.
Figures







Similar articles
-
Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo.Dev Biol. 2006 Aug 1;296(1):104-18. doi: 10.1016/j.ydbio.2006.04.442. Dev Biol. 2006. PMID: 16765933 Free PMC article.
-
BMP-Smad 1/5/8 signalling in the development of the nervous system.Prog Neurobiol. 2013 Oct;109:28-41. doi: 10.1016/j.pneurobio.2013.07.002. Epub 2013 Jul 24. Prog Neurobiol. 2013. PMID: 23891815 Review.
-
Smad signaling in mesenchymal and chondroprogenitor cells.J Bone Joint Surg Am. 2003;85-A Suppl 3:13-8. doi: 10.2106/00004623-200300003-00004. J Bone Joint Surg Am. 2003. PMID: 12925604
-
Common partner Smad-independent canonical bone morphogenetic protein signaling in the specification process of the anterior rhombic lip during cerebellum development.Mol Cell Biol. 2013 May;33(10):1925-37. doi: 10.1128/MCB.01143-12. Epub 2013 Mar 4. Mol Cell Biol. 2013. PMID: 23459943 Free PMC article.
-
Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad.Cytokine Growth Factor Rev. 2009 Oct-Dec;20(5-6):357-65. doi: 10.1016/j.cytogfr.2009.10.017. Epub 2009 Nov 5. Cytokine Growth Factor Rev. 2009. PMID: 19896409 Free PMC article. Review.
Cited by
-
Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development.J Bone Miner Res. 2012 Aug;27(8):1680-94. doi: 10.1002/jbmr.1639. J Bone Miner Res. 2012. PMID: 22508079 Free PMC article.
-
Transcription Factors in Cartilage Homeostasis and Osteoarthritis.Biology (Basel). 2020 Sep 14;9(9):290. doi: 10.3390/biology9090290. Biology (Basel). 2020. PMID: 32937960 Free PMC article. Review.
-
Metabolic reprogramming in skeletal cell differentiation.Bone Res. 2024 Oct 11;12(1):57. doi: 10.1038/s41413-024-00374-0. Bone Res. 2024. PMID: 39394187 Free PMC article. Review.
-
Methotrexate inhibits BMP4 and abrogates the hypertrophic chondrocyte phenotype of synovial fibroblasts in juvenile idiopathic arthritis.Pediatr Rheumatol Online J. 2024 Jan 2;22(1):6. doi: 10.1186/s12969-023-00940-6. Pediatr Rheumatol Online J. 2024. PMID: 38166938 Free PMC article.
-
Combination therapeutics in complex diseases.J Cell Mol Med. 2016 Dec;20(12):2231-2240. doi: 10.1111/jcmm.12930. Epub 2016 Sep 7. J Cell Mol Med. 2016. PMID: 27605177 Free PMC article. Review.
References
-
- Bell, D. M., Leung, K. K., Wheatley, S. C., Ng, L. J., Zhou, S., Ling, K. W., Sham, M. H., Koopman, P., Tam, P. P. and Cheah, K. S. (1997). SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174-178. - PubMed
-
- Bettoun, J. D., Minagawa, M., Kwan, M. Y., Lee, H. S., Yasuda, T., Hendy, G. N., Goltzman, D. and White, J. H. (1997). Cloning and characterization of the promoter regions of the human parathyroid hormone (PTH)/PTH-related peptide receptor gene: analysis of deoxyribonucleic acid from normal subjects and patients with pseudohypoparathyroidism type 1b. J. Clin. Endocrinol. Metab. 82, 1031-1040. - PubMed
-
- Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R. and de Crombrugghe, B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85-89. - PubMed
-
- Brugger, S. M., Merrill, A. E., Torres-Vazquez, J., Wu, N., Ting, M. C., Cho, J. Y., Dobias, S. L., Yi, S. E., Lyons, K., Bell, J. R. et al. (2004). A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131, 5153-5165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

