KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin
- PMID: 19208745
- PMCID: PMC2708114
- DOI: 10.1158/1541-7786.MCR-08-0206
KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin
Abstract
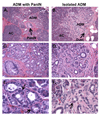
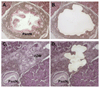
Pancreatic intraepithelial neoplasia (PanIN) is a precursor to invasive ductal adenocarcinoma of the pancreas. Observations made in genetically engineered mouse models suggest that the acinar/centroacinar compartment can give rise to ductal neoplasia. To integrate findings in mice and men, we examined human acinar cells, acinar-ductal metaplasia (ADM) lesions, and PanINs for KRAS2 gene mutations. Surgically resected pancreata were screened for foci of ADM with or without an associated PanIN lesion. Stromal cells, acinar cells, ADMs, and PanINs were separately isolated using laser capture microdissection. KRAS2 status was analyzed using genomic DNA isolated from the microdissected tissue. Twelve of these 31 foci of ADM occurred in isolation, whereas 19 were in the same lobules as a PanIN lesion. All 31 microdissected foci of acinar cells were KRAS2 gene wild-type, as were all 12 isolated ADM lesions lacking an associated PanIN. KRAS2 gene mutations were present in 14 of 19 (74%) PanIN lesions and in 12 of the 19 (63%) foci of ADM associated with these PanINs. All ADM lesions with a KRAS2 gene mutation harbored the identical KRAS2 gene mutation found in their associated PanIN lesions. Ductal neoplasms of the human pancreas, as defined by KRAS2 gene mutations, do not appear to arise from acinar cells. Isolated AMD lesions are genetically distinct from those associated with PanINs, and the latter may represent retrograde extension of the neoplastic PanIN cells or less likely are precursors to PanIN.
Figures

Similar articles
-
Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia.Oncogene. 2013 Apr 11;32(15):1950-8. doi: 10.1038/onc.2012.210. Epub 2012 Jun 4. Oncogene. 2013. PMID: 22665051 Free PMC article.
-
Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias.Mod Pathol. 2011 Feb;24(2):256-66. doi: 10.1038/modpathol.2010.181. Epub 2010 Sep 24. Mod Pathol. 2011. PMID: 20871595 Free PMC article.
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
Acinar-to-Ductal Metaplasia (ADM): On the Road to Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Cancer.Int J Mol Sci. 2023 Jun 9;24(12):9946. doi: 10.3390/ijms24129946. Int J Mol Sci. 2023. PMID: 37373094 Free PMC article. Review.
-
Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs).Langenbecks Arch Surg. 2008 Jul;393(4):561-70. doi: 10.1007/s00423-008-0282-x. Epub 2008 Feb 19. Langenbecks Arch Surg. 2008. PMID: 18283486 Free PMC article. Review.
Cited by
-
A Small Molecule with Big Impact: MRTX1133 Targets the KRASG12D Mutation in Pancreatic Cancer.Clin Cancer Res. 2024 Feb 16;30(4):655-662. doi: 10.1158/1078-0432.CCR-23-2098. Clin Cancer Res. 2024. PMID: 37831007 Free PMC article.
-
Mutational analysis of cytocentrifugation supernatant fluid from pancreatic solid mass lesions.Diagn Cytopathol. 2014 Aug;42(8):719-25. doi: 10.1002/dc.23048. Epub 2013 Nov 22. Diagn Cytopathol. 2014. PMID: 24265269 Free PMC article.
-
Developmental Pathways Direct Pancreatic Cancer Initiation from Its Cellular Origin.Stem Cells Int. 2016;2016:9298535. doi: 10.1155/2016/9298535. Epub 2015 Nov 22. Stem Cells Int. 2016. PMID: 26681957 Free PMC article. Review.
-
Comprehensive DNA Methylation Analysis Indicates That Pancreatic Intraepithelial Neoplasia Lesions Are Acinar-Derived and Epigenetically Primed for Carcinogenesis.Cancer Res. 2023 Jun 2;83(11):1905-1916. doi: 10.1158/0008-5472.CAN-22-4052. Cancer Res. 2023. PMID: 36989344 Free PMC article.
-
Pancreatic acinar cell carcinoma with a ductal adenocarcinoma component: a case report and analysis of the histogenesis of the tumor.World J Surg Oncol. 2020 Sep 5;18(1):238. doi: 10.1186/s12957-020-02014-3. World J Surg Oncol. 2020. PMID: 32891173 Free PMC article.
References
-
- Hulst SPL. Zur kenntinis der Genese des Adenokarzinoms und Karzinoms des Pankreas. Virchows Arch (B) 1905;180:288–316.
-
- Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690–2698. - PubMed
-
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. - PubMed
-
- Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

