Adenovirus-mediated hPNPase(old-35) gene transfer as a therapeutic strategy for neuroblastoma
- PMID: 19202553
- PMCID: PMC2921678
- DOI: 10.1002/jcp.21719
Adenovirus-mediated hPNPase(old-35) gene transfer as a therapeutic strategy for neuroblastoma
Abstract
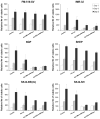
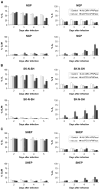
Current treatment options for neuroblastoma fail to eradicate the disease in the majority of high-risk patients, clearly mandating development of innovative therapeutic strategies. Gene therapy represents a promising approach for reversing the neoplastic phenotype or driving tumor cells to self-destruction. We presently studied the effects of adenovirus-mediated gene transfer of human polynucleotide phosphorylase (hPNPase(old-35)), a 3',5'-exoribonuclease with growth-inhibitory properties, in neuroblastoma cells. Transgene expression was driven by either the cytomegalovirus (CMV) promoter or by a tumor-selective promoter derived from progression elevated gene-3 (PEG-3). Our data demonstrate that efficient adenoviral transduction of neuroblastoma cells and robust transgene expression are feasible objectives, that the PEG-3 promoter is capable of selectively targeting gene expression in the majority of neuroblastoma cells, and that hPNPase(old-35) induces profound growth suppression and apoptosis of malignant neuroblastoma cells, while exerting limited effects on normal neural crest-derived melanocytes. These findings support future applications of hPNPase(old-35) for targeted gene-based therapy of neuroblastoma and suggest that combination with the PEG-3 promoter holds promise for creating a potent and selective neuroblastoma therapeutic. J. Cell. Physiol. 219: 707-715, 2009. (c) 2009 Wiley-Liss, Inc.
Figures
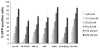

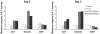
Similar articles
-
Progression elevated gene-3 promoter (PEG-Prom) confers cancer cell selectivity to human polynucleotide phosphorylase (hPNPase(old-35))-mediated growth suppression.J Cell Physiol. 2008 May;215(2):401-9. doi: 10.1002/jcp.21320. J Cell Physiol. 2008. PMID: 17960560
-
Expression regulation and genomic organization of human polynucleotide phosphorylase, hPNPase(old-35), a Type I interferon inducible early response gene.Gene. 2003 Oct 16;316:143-56. doi: 10.1016/s0378-1119(03)00752-2. Gene. 2003. PMID: 14563561
-
Defining the mechanism by which IFN-beta dowregulates c-myc expression in human melanoma cells: pivotal role for human polynucleotide phosphorylase (hPNPaseold-35).Cell Death Differ. 2006 Sep;13(9):1541-53. doi: 10.1038/sj.cdd.4401829. Epub 2006 Jan 13. Cell Death Differ. 2006. PMID: 16410805
-
Transductional and transcriptional targeting of adenovirus for clinical applications.Curr Gene Ther. 2004 Mar;4(1):1-14. doi: 10.2174/1566523044577997. Curr Gene Ther. 2004. PMID: 15032610 Review.
-
Human polynucleotide phosphorylase (hPNPase old-35): an RNA degradation enzyme with pleiotrophic biological effects.Cell Cycle. 2006 May;5(10):1080-4. doi: 10.4161/cc.5.10.2741. Epub 2006 May 15. Cell Cycle. 2006. PMID: 16687933 Review.
Cited by
-
Ribonucleases and bacterial virulence.Microb Biotechnol. 2011 Sep;4(5):558-71. doi: 10.1111/j.1751-7915.2010.00212.x. Epub 2010 Oct 15. Microb Biotechnol. 2011. PMID: 21375713 Free PMC article. Review.
-
Gene therapy as a potential tool for treating neuroblastoma-a focused review.Cancer Gene Ther. 2016 May;23(5):115-24. doi: 10.1038/cgt.2016.16. Epub 2016 Apr 15. Cancer Gene Ther. 2016. PMID: 27080224 Review.
-
Recombinant adenovirus encoding vasohibin prevents tumor angiogenesis and inhibits tumor growth.Cancer Sci. 2010 Feb;101(2):448-52. doi: 10.1111/j.1349-7006.2009.01388.x. Epub 2009 Oct 6. Cancer Sci. 2010. PMID: 19886910 Free PMC article.
-
Killing of cancer cells through the use of eukaryotic expression vectors harbouring genes encoding nucleases and ribonuclease inhibitor.Tumour Biol. 2015 May;36(5):3147-57. doi: 10.1007/s13277-015-3360-z. Epub 2015 Apr 1. Tumour Biol. 2015. PMID: 25874497 Review.
-
Human polynucleotide phosphorylase selectively and preferentially degrades microRNA-221 in human melanoma cells.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11948-53. doi: 10.1073/pnas.0914143107. Epub 2010 Jun 14. Proc Natl Acad Sci U S A. 2010. PMID: 20547861 Free PMC article.
References
-
- Ardelt B, Ardelt W, Darzynkiewicz Z. Cytotoxic ribonucleases and RNA interference (RNAi) Cell Cycle. 2003;2:22–24. - PubMed
-
- Arnold U, Ulbrich-Hofmann R. Natural and engineered ribonucleases as potential cancer therapeutics. Biotechnol Lett. 2006;28:1615–1622. - PubMed
-
- Beck AK, Pass HI, Carbone M, Yang H. Ranpirnase as a potential antitumor ribonuclease treatment for mesothelioma and other malignancies. Future Oncol. 2008;4:341–349. - PubMed
-
- Benito A, Ribo M, Vilanova M. On the track of antitumour ribonucleases. Mol Biosyst. 2005;1:294–302. - PubMed
-
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA102321/CA/NCI NIH HHS/United States
- P01 CA104177-05/CA/NCI NIH HHS/United States
- P01 CA104177-01A2/CA/NCI NIH HHS/United States
- P01 CA104177-04/CA/NCI NIH HHS/United States
- R01 CA035675/CA/NCI NIH HHS/United States
- R01 CA035675-20/CA/NCI NIH HHS/United States
- P01 CA104177-040001/CA/NCI NIH HHS/United States
- P01 CA104177-020001/CA/NCI NIH HHS/United States
- R01 CA035675-21/CA/NCI NIH HHS/United States
- P01 CA104177-02/CA/NCI NIH HHS/United States
- P01 CA104177-030001/CA/NCI NIH HHS/United States
- P01 CA104177-050001/CA/NCI NIH HHS/United States
- R01 CA102321-08/CA/NCI NIH HHS/United States
- R01 CA035675-22/CA/NCI NIH HHS/United States
- P01 CA104177-03/CA/NCI NIH HHS/United States
- P01 CA104177-01A20001/CA/NCI NIH HHS/United States
- R01 CA102321-06/CA/NCI NIH HHS/United States
- R01 CA102321-07/CA/NCI NIH HHS/United States
- R01 CA035675-19/CA/NCI NIH HHS/United States
- P01 CA104177/CA/NCI NIH HHS/United States
- R01 CA102321-04A1/CA/NCI NIH HHS/United States
- R01 CA035675-18/CA/NCI NIH HHS/United States
- R01 CA102321-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

