Knockdown of caveolin-1 by siRNA inhibits the transformation of mouse hepatoma H22 cells in vitro and in vivo
- PMID: 19196097
- PMCID: PMC2948472
- DOI: 10.1089/oli.2008.0166
Knockdown of caveolin-1 by siRNA inhibits the transformation of mouse hepatoma H22 cells in vitro and in vivo
Abstract
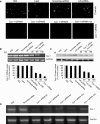
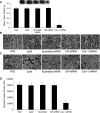
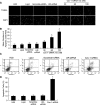
Caveolin-1 (Cav-1) is a main structural protein of caveolae and plays important roles in signal transduction and tumorigenesis. We previously showed that Cav-1 was highly expressed in mouse hepatoma cell lines and positively correlated with cell invasion capability. Thus, interfering with the expression and activity of Cav-1 might be a potential way to intervene with hepatoma progression. We used RNA interference to study the biological effects of silencing Cav-1 expression in hepatoma H22 cells, to validate its potential as a therapeutic target. Using small-interfering RNAs (siRNAs) targeting the mRNA region of Cav-1, we effectively suppressed Cav-1 mRNA and protein levels. This resulted in the decreased transformation ability of H22 cells in vitro and in vivo. In addition, downregulation of Cav-1 expression promoted the apoptosis of H22 cells in vitro and in vivo. These results suggest that the use of siRNA could be an efficient molecular therapeutic method for hepatoma with high expression of Cav-1.
Figures
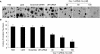
Similar articles
-
Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice.J Biol Chem. 2005 Jul 1;280(26):25134-45. doi: 10.1074/jbc.M501186200. Epub 2005 Mar 30. J Biol Chem. 2005. PMID: 15802273
-
Upregulation of Caveolin-1 correlate with Akt expression and poor prognosis in NPC patients.Laryngoscope. 2015 Jul;125(7):E231-8. doi: 10.1002/lary.25297. Epub 2015 Apr 15. Laryngoscope. 2015. PMID: 25877996
-
Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells.Radiother Oncol. 2009 Sep;92(3):362-70. doi: 10.1016/j.radonc.2009.07.004. Epub 2009 Aug 6. Radiother Oncol. 2009. PMID: 19665245
-
Caveolin-1 promotes the transformation and anti-apoptotic ability of mouse hepatoma cells.IUBMB Life. 2008 Oct;60(10):693-9. doi: 10.1002/iub.104. IUBMB Life. 2008. PMID: 18618588
-
Expression of Caveolin-1 in Periodontal Tissue and Its Role in Osteoblastic and Cementoblastic Differentiation In Vitro.Calcif Tissue Int. 2016 May;98(5):497-510. doi: 10.1007/s00223-015-0095-1. Epub 2015 Dec 19. Calcif Tissue Int. 2016. PMID: 26686692
Cited by
-
Design, Synthesis and Evaluation of Naphthalimide Derivatives as Potential Anticancer Agents for Hepatocellular Carcinoma.Molecules. 2017 Feb 22;22(2):342. doi: 10.3390/molecules22020342. Molecules. 2017. PMID: 28241441 Free PMC article.
-
Association of caveolin-1 protein expression with hepatocellular carcinoma: a meta-analysis and literature review.Cancer Manag Res. 2019 May 31;11:5113-5122. doi: 10.2147/CMAR.S194033. eCollection 2019. Cancer Manag Res. 2019. PMID: 31239768 Free PMC article.
-
Reciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepatocellular carcinoma.PLoS One. 2014 Aug 22;9(8):e105278. doi: 10.1371/journal.pone.0105278. eCollection 2014. PLoS One. 2014. PMID: 25148256 Free PMC article.
-
Mechanosensory entities and functionality of endothelial cells.Front Cell Dev Biol. 2024 Oct 23;12:1446452. doi: 10.3389/fcell.2024.1446452. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39507419 Free PMC article. Review.
References
-
- ANDERSON R.G. JACOBSON K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
-
- BENDER F.C. REYMOND M.A. BRON C. QUEST A.F. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. - PubMed
-
- COX A.D. DER C.J. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. - PubMed
-
- DRAB M. VERKADE P. ELGER M. KASPER M. LOHN M. LAUTERBACH B. MENNE J. LINDSCHAU C. MENDE F. LUFT F.C. SCHEDL A. HALLER H. KURZCHALIA T.V. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. - PubMed
-
- ENGELMAN J.A. LEE R.J. KARNEZIS A. BEARSS D.J. WEBSTER M. SIEGEL P. MULLER W.J. WINDLE J.J. PESTELL R.G. LISANTI M.P. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J. Biol. Chem. 1998;273:20448–20455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

