Getting the iron out: phlebotomy for Alzheimer's disease?
- PMID: 19195795
- PMCID: PMC2732125
- DOI: 10.1016/j.mehy.2008.12.029
Getting the iron out: phlebotomy for Alzheimer's disease?
Abstract
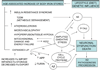
This communication explores the temporal link between the age-associated increase in body iron stores and the age-related incidence of Alzheimer's disease (AD), the most prevalent cause of senile dementia. Body iron stores that increase with age could be pivotal to AD pathogenesis and progression. Increased stored iron is associated with common medical conditions such as diabetes and vascular disease that increase risk for development of AD. Increased stored iron could also promote oxidative stress/free radical damage in vulnerable neurons, a critical early change in AD. A ferrocentric model of AD described here forms the basis of a rational, easily testable experimental therapeutic approach for AD, which if successful, would be both widely applicable and inexpensive. Clinical studies have shown that calibrated phlebotomy is an effective way to reduce stored iron safely and predictably without causing anemia. We hypothesize that reducing stored iron by calibrated phlebotomy to avoid iron deficiency will improve cerebrovascular function, slow neurodegenerative change, and improve cognitive and behavioral functions in AD. The hypothesis is eminently testable as iron reduction therapy is useful for chronic diseases associated with iron excess such as nonalcoholic steatohepatitis (NASH), atherosclerosis, hereditary hemochromatosis and thalassemia. Testing this hypothesis could provide valuable insight into the causation of AD and suggest novel preventive and treatment strategies.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Similar articles
-
Role of stored iron in atherosclerosis.J Vasc Nurs. 2000 Dec;18(4):109-14; quiz 115-6. doi: 10.1067/mvn.2000.111614. J Vasc Nurs. 2000. PMID: 11995291 Review.
-
Blood removal therapy in hereditary hemochromatosis induces a stress response resulting in improved genome integrity.Transfusion. 2016 Jun;56(6):1435-41. doi: 10.1111/trf.13588. Epub 2016 Apr 4. Transfusion. 2016. PMID: 27045387
-
Iron Overload in the Liver of 2 Children: Nonalcoholic Steatohepatitis and Juvenile Hemochromatosis.J Pediatr Hematol Oncol. 2017 Aug;39(6):466-469. doi: 10.1097/MPH.0000000000000752. J Pediatr Hematol Oncol. 2017. PMID: 28067690
-
Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial.JAMA. 2007 Feb 14;297(6):603-10. doi: 10.1001/jama.297.6.603. JAMA. 2007. PMID: 17299195 Clinical Trial.
-
Management of hemochromatosis. Hemochromatosis Management Working Group.Ann Intern Med. 1998 Dec 1;129(11):932-9. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. Ann Intern Med. 1998. PMID: 9867745 Review.
Cited by
-
Expression of Iron Transporters and Pathological Hallmarks of Parkinson's and Alzheimer's Diseases in the Brain of Young, Adult, and Aged Rats.Mol Neurobiol. 2017 Sep;54(7):5213-5224. doi: 10.1007/s12035-016-0067-0. Epub 2016 Aug 30. Mol Neurobiol. 2017. PMID: 27578012
-
Candidate SNP Markers of Familial and Sporadic Alzheimer's Diseases Are Predicted by a Significant Change in the Affinity of TATA-Binding Protein for Human Gene Promoters.Front Aging Neurosci. 2017 Jul 20;9:231. doi: 10.3389/fnagi.2017.00231. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28775688 Free PMC article.
-
A Scoping Review of Alzheimers Disease Hypotheses: An Array of Uni- and Multi-Factorial Theories.J Alzheimers Dis. 2024;99(3):843-856. doi: 10.3233/JAD-230772. J Alzheimers Dis. 2024. PMID: 38788067 Free PMC article. Review.
-
Metal dyshomeostasis and inflammation in Alzheimer's and Parkinson's diseases: possible impact of environmental exposures.Oxid Med Cell Longev. 2013;2013:726954. doi: 10.1155/2013/726954. Epub 2013 Apr 17. Oxid Med Cell Longev. 2013. PMID: 23710288 Free PMC article. Review.
-
Dalteparin as a Novel Therapeutic Agent to Prevent Diabetic Encephalopathy by Targeting Oxidative Stress and Inflammation.Basic Clin Neurosci. 2020 Nov-Dec;11(6):795-804. doi: 10.32598/bcn.11.6.1775.1. Epub 2020 Nov 1. Basic Clin Neurosci. 2020. PMID: 33850616 Free PMC article.
References
-
- Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26:207–214. - PubMed
-
- Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2006;17:93–100. - PubMed
-
- Sullivan JL. Iron and the sex difference in heart disease rate. Lancet. 1981;1:1293–1294. - PubMed
-
- Sullivan JL. Misconceptions in the debate on the iron hypothesis. J Nutr Biochem. 2001;12:33–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

