The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion
- PMID: 19188498
- PMCID: PMC2646575
- DOI: 10.1084/jem.20081829
The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion
Abstract
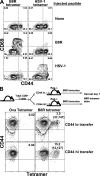
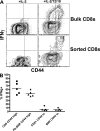
Memory T cells exhibit superior responses to pathogens and tumors compared with their naive counterparts. Memory is typically generated via an immune response to a foreign antigen, but functional memory T cells can also be produced from naive cells by homeostatic mechanisms. Using a recently developed method, we studied CD8 T cells, which are specific for model (ovalbumin) and viral (HSV, vaccinia) antigens, in unimmunized mice and found a subpopulation bearing markers of memory cells. Based on their phenotypic markers and by their presence in germ-free mice, these preexisting memory-like CD44(hi) CD8 T cells are likely to arise via physiological homeostatic proliferation rather than a response to environmental microbes. These antigen-inexperienced memory phenotype CD8 T cells display several functions that distinguish them from their CD44(lo) counterparts, including a rapid initiation of proliferation after T cell stimulation and rapid IFN-gamma production after exposure to proinflammatory cytokines. Collectively, these data indicate that the unprimed antigen-specific CD8 T cell repertoire contains antigen-inexperienced cells that display phenotypic and functional traits of memory cells.
Keywords: Virtual Memory.
Figures





Similar articles
-
Differential interferon-γ production by naive and memory-like CD8 T cells.J Leukoc Biol. 2020 Oct;108(4):1329-1337. doi: 10.1002/JLB.2AB0420-646R. Epub 2020 May 18. J Leukoc Biol. 2020. PMID: 32421902
-
CD8+, but not CD4+ effector/memory T cells, express the CD44highCD45RBhigh phenotype with aging, which displays reduced expression levels of P2X7 receptor and ATP-induced cellular responses.FASEB J. 2019 Mar;33(3):3225-3236. doi: 10.1096/fj.201800867R. Epub 2018 Nov 1. FASEB J. 2019. PMID: 30383448
-
Reevaluation of the origin of CD44(high) "memory phenotype" CD8 T cells: comparison between memory CD8 T cells and thymus-independent CD8 T cells.Eur J Immunol. 2001 Jun;31(6):1917-26. doi: 10.1002/1521-4141(200106)31:6<1917::aid-immu1917>3.0.co;2-f. Eur J Immunol. 2001. PMID: 11433389
-
Opinion: Virtual memory CD8 T cells and lymphopenia-induced memory CD8 T cells represent a single subset: Homeostatic memory T cells.Immunol Lett. 2018 Nov;203:57-61. doi: 10.1016/j.imlet.2018.09.003. Epub 2018 Sep 20. Immunol Lett. 2018. PMID: 30243945 Review.
-
Striking a Balance-Cellular and Molecular Drivers of Memory T Cell Development and Responses to Chronic Stimulation.Front Immunol. 2019 Jul 17;10:1595. doi: 10.3389/fimmu.2019.01595. eCollection 2019. Front Immunol. 2019. PMID: 31379821 Free PMC article. Review.
Cited by
-
Streptococcus agalactiae and Escherichia coli induce distinct effector γδ T cell responses during neonatal sepsis.iScience. 2024 Apr 5;27(5):109669. doi: 10.1016/j.isci.2024.109669. eCollection 2024 May 17. iScience. 2024. PMID: 38646164 Free PMC article.
-
Is the exquisite specificity of lymphocytes generated by thymic selection or due to evolution?Front Immunol. 2024 Mar 25;15:1266349. doi: 10.3389/fimmu.2024.1266349. eCollection 2024. Front Immunol. 2024. PMID: 38605941 Free PMC article.
-
Unique immune profiles in collaborative cross mice linked to survival and viral clearance upon infection.iScience. 2024 Feb 2;27(3):109103. doi: 10.1016/j.isci.2024.109103. eCollection 2024 Mar 15. iScience. 2024. PMID: 38361611 Free PMC article.
-
PREX1 improves homeostatic proliferation to maintain a naive CD4+ T cell compartment in older age.JCI Insight. 2024 Feb 8;9(5):e172848. doi: 10.1172/jci.insight.172848. JCI Insight. 2024. PMID: 38329813 Free PMC article.
-
Alternatively Spliced Variants of Murine CD247 Influence T Cell Development and Activation, Revealing the Importance of the CD3ζ C-Terminal Region.J Immunol. 2024 Feb 15;212(4):541-550. doi: 10.4049/jimmunol.2300511. J Immunol. 2024. PMID: 38117282
References
-
- Harty J.T., Badovinac V.P. 2008. Shaping and reshaping CD8+ T-cell memory.Nat. Rev. Immunol. 8:107–119 - PubMed
-
- Seder R.A., Ahmed R. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation.Nat. Immunol. 4:835–842 - PubMed
-
- Jameson S.C. 2005. T cell homeostasis: keeping useful T cells alive and live T cells useful.Semin. Immunol. 17:231–237 - PubMed
-
- Marleau A.M., Sarvetnick N. 2005. T cell homeostasis in tolerance and immunity.J. Leukoc. Biol. 78:575–584 - PubMed
-
- Surh C.D., Sprent J. 2005. Regulation of mature T cell homeostasis.Semin. Immunol. 17:183–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

