Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy
- PMID: 19187773
- PMCID: PMC2673980
- DOI: 10.1016/j.cmet.2009.01.002
Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy
Abstract
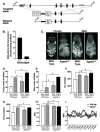
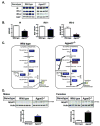
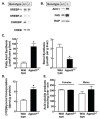
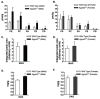
Mutations in 1-acylglycerol-3-phosphate-O-acyltransferase 2 (AGPAT2) cause congenital generalized lipodystrophy. To understand the molecular mechanisms underlying the metabolic complications associated with AGPAT2 deficiency, Agpat2 null mice were generated. Agpat2(-/-) mice develop severe lipodystrophy affecting both white and brown adipose tissue, extreme insulin resistance, diabetes, and hepatic steatosis. The expression of lipogenic genes and rates of de novo fatty acid biosynthesis were increased approximately 4-fold in Agpat2(-/-) mouse livers. The mRNA and protein levels of monoacylglycerol acyltransferase isoform 1 were markedly increased in the livers of Agpat2(-/-) mice, suggesting that the alternative monoacylglycerol pathway for triglyceride biosynthesis is activated in the absence of AGPAT2. Feeding a fat-free diet reduced liver triglycerides by approximately 50% in Agpat2(-/-) mice. These observations suggest that both dietary fat and hepatic triglyceride biosynthesis via a monoacylglycerol pathway may contribute to hepatic steatosis in Agpat2(-/-) mice.
Figures
Similar articles
-
Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice.J Biol Chem. 2011 Oct 28;286(43):37676-91. doi: 10.1074/jbc.M111.250449. Epub 2011 Aug 27. J Biol Chem. 2011. PMID: 21873652 Free PMC article.
-
Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2-/- lipodystrophic mice independent of hepatocyte leptin receptors.J Lipid Res. 2014 Feb;55(2):276-88. doi: 10.1194/jlr.M045799. Epub 2013 Nov 30. J Lipid Res. 2014. PMID: 24293639 Free PMC article.
-
Pathology of congenital generalized lipodystrophy in Agpat2-/- mice.Vet Pathol. 2011 May;48(3):642-54. doi: 10.1177/0300985810383870. Epub 2010 Nov 4. Vet Pathol. 2011. PMID: 21051554
-
Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways.Trends Endocrinol Metab. 2003 Jul;14(5):214-21. doi: 10.1016/s1043-2760(03)00078-x. Trends Endocrinol Metab. 2003. PMID: 12826327 Review.
-
Genetic basis of congenital generalized lipodystrophy.Int J Obes Relat Metab Disord. 2004 Feb;28(2):336-9. doi: 10.1038/sj.ijo.0802487. Int J Obes Relat Metab Disord. 2004. PMID: 14557833 Review.
Cited by
-
The Multifaceted Roles of Adipose Tissue-Therapeutic Targets for Diabetes and Beyond: The 2015 Banting Lecture.Diabetes. 2016 Jun;65(6):1452-61. doi: 10.2337/db16-0339. Diabetes. 2016. PMID: 27222389 Free PMC article.
-
Adipose-specific overexpression of human AGPAT2 in mice causes increased adiposity and mild hepatic dysfunction.iScience. 2023 Dec 7;27(1):108653. doi: 10.1016/j.isci.2023.108653. eCollection 2024 Jan 19. iScience. 2023. PMID: 38274405 Free PMC article.
-
New insight into the role of MMP14 in metabolic balance.PeerJ. 2016 Jul 13;4:e2142. doi: 10.7717/peerj.2142. eCollection 2016. PeerJ. 2016. PMID: 27478693 Free PMC article.
-
Enzymatic activity of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 11: upregulated in breast and cervical cancers.J Lipid Res. 2010 Aug;51(8):2143-52. doi: 10.1194/jlr.M004762. Epub 2010 Apr 2. J Lipid Res. 2010. PMID: 20363836 Free PMC article.
-
Studies of association of AGPAT6 variants with type 2 diabetes and related metabolic phenotypes in 12,068 Danes.BMC Med Genet. 2013 Oct 25;14:113. doi: 10.1186/1471-2350-14-113. BMC Med Genet. 2013. PMID: 24156295 Free PMC article.
References
-
- Agarwal AK, Arioglu E, de Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. - PubMed
-
- Agarwal AK, Barnes RI, Garg A. Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: cloning, tissue distribution, gene structure and enzymatic activity. Arch Biochem Biophys. 2006;449:64–76. - PubMed
-
- Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab. 2003;14:214–221. - PubMed
-
- Agarwal AK, Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet. 2006;7:175–199. - PubMed
-
- Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NHLB, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88:4840–4847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

