Adsorption of GST-PI3Kgamma at the air-buffer interface and at substrate and nonsubstrate phospholipid monolayers
- PMID: 19186139
- PMCID: PMC2716572
- DOI: 10.1016/j.bpj.2008.10.032
Adsorption of GST-PI3Kgamma at the air-buffer interface and at substrate and nonsubstrate phospholipid monolayers
Abstract
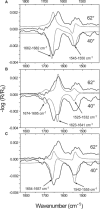
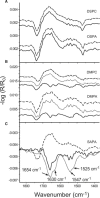
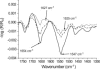
The recruitment of phosphoinositide 3-kinase gamma (PI3Kgamma) to the cell membrane is a crucial requirement for the initiation of inflammation cascades by second-messenger production. In addition to identifying other regulation pathways, it has been found that PI3Kgamma is able to bind phospholipids directly. In this study, the adsorption behavior of glutathione S-transferase (GST)-PI3Kgamma to nonsubstrate model phospholipids, as well as to commercially available substrate inositol phospholipids (phosphoinositides), was investigated by use of infrared reflection-absorption spectroscopy (IRRAS). The nonsubstrate phospholipid monolayers also yielded important information about structural requirements for protein adsorption. The enzyme did not interact with condensed zwitterionic or anionic monolayers; however, it could penetrate into uncompressed fluid monolayers. Compression to values above its equilibrium pressure led to a squeezing out and desorption of the protein. Protein affinity for the monolayer surface increased considerably when the lipid had an anionic headgroup and contained an arachidonoyl fatty acyl chain in sn-2 position. Similar results on a much higher level were observed with substrate phosphoinositides. No structural response of GST-PI3Kgamma to lipid interaction was detected by IRRAS. On the other hand, protein adsorption caused a condensing effect in phosphoinositide monolayers. In addition, the protein reduced the charge density at the interface probably by shifting the pK values of the phosphate groups attached to the inositol headgroups. Because of their strongly polar headgroups, an interaction of the inositides with the water molecules of the subphase can be expected. This interaction is disturbed by protein adsorption, causing the ionization state of the phosphates to change.
Figures
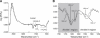
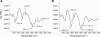
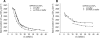
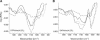
Similar articles
-
Adsorption of amyloid beta (1-40) peptide at phospholipid monolayers.Chembiochem. 2005 Oct;6(10):1817-24. doi: 10.1002/cbic.200500116. Chembiochem. 2005. PMID: 16175542
-
Adsorption of a phospholipid-hydroperoxide glutathione peroxidase into phospholipid monolayers at the air-water interface.Colloids Surf B Biointerfaces. 2004 May 15;35(2):99-105. doi: 10.1016/j.colsurfb.2004.02.011. Colloids Surf B Biointerfaces. 2004. PMID: 15261042
-
Interaction of the neurotransmitter, neuropeptide Y, with phospholipid membranes: infrared spectroscopic characterization at the air/water interface.J Phys Chem B. 2006 Nov 9;110(44):22152-9. doi: 10.1021/jp062537q. J Phys Chem B. 2006. PMID: 17078651 Free PMC article.
-
PI3K class IB pathway.Sci STKE. 2007 Oct 9;2007(407):cm2. doi: 10.1126/stke.4072007cm2. Sci STKE. 2007. PMID: 17925573 Review.
-
Phosphoinositide 3-kinase gamma (PI3Kgamma) inhibitors for the treatment of inflammation and autoimmune disease.Recent Pat Inflamm Allergy Drug Discov. 2010 Jan;4(1):1-15. doi: 10.2174/187221310789895603. Recent Pat Inflamm Allergy Drug Discov. 2010. PMID: 20017720 Review.
Cited by
-
Binding of the GTPase Sar1 to a Lipid Membrane Monolayer: Insertion and Orientation Studied by Infrared Reflection⁻Absorption Spectroscopy.Polymers (Basel). 2017 Nov 14;9(11):612. doi: 10.3390/polym9110612. Polymers (Basel). 2017. PMID: 30965916 Free PMC article.
-
Biophysical methods for the characterization of PTEN/lipid bilayer interactions.Methods. 2015 May;77-78:125-35. doi: 10.1016/j.ymeth.2015.02.004. Epub 2015 Feb 16. Methods. 2015. PMID: 25697761 Free PMC article. Review.
-
Improved functional recovery to I/R injury in hearts from lipocalin-2 deficiency mice: restoration of mitochondrial function and phospholipids remodeling.Am J Transl Res. 2012;4(1):60-71. Epub 2012 Jan 5. Am J Transl Res. 2012. PMID: 22355443 Free PMC article.
References
-
- Martin T.F.J. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 1998;14:231–264. - PubMed
-
- Rameh L.E., Cantley L.C. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 1999;274:8347–8350. - PubMed
-
- Vanhaesebroeck B., Jones G.E., Allen W.E., Zicha D., Hooshmand-Rad R. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat. Cell Biol. 1999;1:69–71. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

