Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice
- PMID: 19164485
- PMCID: PMC2670665
- DOI: 10.1152/ajpgi.90507.2008
Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice
Abstract
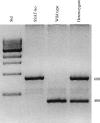
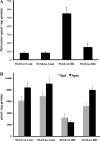
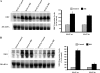
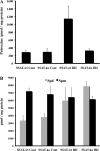
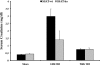
Expression of spermine/spermidine-N1-acetyltransferase (SSAT), the rate-limiting enzyme of polyamine backconversion cascade, increases after ischemia-reperfusion injuries (IRI). We hypothesized that SSAT plays an important role in the mediation of IRI. To test our hypothesis, wild-type (SSAT-wt) and SSAT-deficient (SSAT-ko) mice were subjected to liver or kidney IRI by ligation of hepatic or renal arteries. The liver and kidney content of putrescine (Put), a downstream by-product of SSAT activity, increased in SSAT-wt animals but not in SSAT-ko animals after IRI, indicating that polyamine backconversion is not functional in SSAT-deficient mice. When subjected to hepatic IRI, SSAT-ko mice were significantly protected against liver damage compared with SSAT-wt mice. Similarly, SSAT-ko animals subjected to renal IRI showed significantly greater protection against damage to kidney tubules than SSAT-wt mice. These studies indicate that SSAT-deficient animals are protected against IRI and suggest that SSAT is an important mediator of the tissue damage in IRI.
Figures

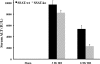



Similar articles
-
The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury.Am J Physiol Cell Physiol. 2010 Jul;299(1):C164-74. doi: 10.1152/ajpcell.00512.2009. Epub 2010 Apr 14. Am J Physiol Cell Physiol. 2010. PMID: 20392931 Free PMC article.
-
Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2003 May;284(5):F1046-55. doi: 10.1152/ajprenal.00318.2002. Epub 2003 Jan 28. Am J Physiol Renal Physiol. 2003. PMID: 12554636
-
Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury.Am J Physiol Cell Physiol. 2005 Oct;289(4):C826-35. doi: 10.1152/ajpcell.00629.2004. Epub 2005 May 11. Am J Physiol Cell Physiol. 2005. PMID: 15888550
-
Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator.Am J Physiol Endocrinol Metab. 2008 Jun;294(6):E995-1010. doi: 10.1152/ajpendo.90217.2008. Epub 2008 Mar 18. Am J Physiol Endocrinol Metab. 2008. PMID: 18349109 Review.
-
The Association between Spermidine/Spermine N1-Acetyltransferase (SSAT) and Human Malignancies.Int J Mol Sci. 2022 May 25;23(11):5926. doi: 10.3390/ijms23115926. Int J Mol Sci. 2022. PMID: 35682610 Free PMC article. Review.
Cited by
-
Spermidine Confers Liver Protection by Enhancing NRF2 Signaling Through a MAP1S-Mediated Noncanonical Mechanism.Hepatology. 2019 Jul;70(1):372-388. doi: 10.1002/hep.30616. Epub 2019 May 20. Hepatology. 2019. PMID: 30873635 Free PMC article.
-
Polyamines and Their Metabolism: From the Maintenance of Physiological Homeostasis to the Mediation of Disease.Med Sci (Basel). 2022 Jul 15;10(3):38. doi: 10.3390/medsci10030038. Med Sci (Basel). 2022. PMID: 35893120 Free PMC article. Review.
-
Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury.PLoS One. 2017 Sep 8;12(9):e0184570. doi: 10.1371/journal.pone.0184570. eCollection 2017. PLoS One. 2017. PMID: 28886181 Free PMC article.
-
A novel assay platform for the detection of translation modulators of spermidine/spermine acetyltransferase.Curr Pharm Des. 2014;20(2):245-52. doi: 10.2174/13816128113199990035. Curr Pharm Des. 2014. PMID: 23701549 Free PMC article.
-
Polyamine Catabolism in Acute Kidney Injury.Int J Mol Sci. 2019 Sep 26;20(19):4790. doi: 10.3390/ijms20194790. Int J Mol Sci. 2019. PMID: 31561575 Free PMC article. Review.
References
-
- Alhonen L, Karppinen A, Uusi-Oukari M, Vujcic S, Korhonen VP, Halmekyto M, Kramer DL, Hines R, Janne J, Porter CW. Correlation of polyamine and growth responses to N1,N11-diethylnorspermine in primary fetal fibroblasts derived from transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 273: 1964–1969, 1998. - PubMed
-
- Barone S, Okaya T, Rudich S, Petrovic S, Tehrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia/reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005. - PubMed
-
- Baskaya MK, Rao AM, Dogan A, Donaldson D, Gellin G, Dempsey RJ. Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res 744: 302–308, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

